All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Avapritinib produces durable responses in patients with advanced systemic mastocytosis
Systemic mastocytosis (SM) is a rare hematologic clonal neoplasm characterized by the infiltration of excessive numbers of mast cells in various organs, resulting in severe pathological features including organ damage and mast cell-related mediator symptoms. 1 The etiology remains uncertain, however mutations of the KIT D816V gene within mast cells present in 95% of cases. Additionally, treatment for advanced SM (AdvSM)—which encompasses aggressive SM (ASM), SM with associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL)—has proven challenging. Patients with these disorders currently have poor prognosis, with a decreased survival.
The development of KIT inhibitors has improved the therapeutic landscape for AdvSM. Midostaurin was the first inhibitor approved by the U.S. Food and Drug Administration (FDA) in 2017 for the treatment of AdvSM in adult patients after demonstrating durable responses. Recently, a second KIT inhibitor, avapritinib, was also approved by the FDA for AdvSM.2 This approval followed results from the phase I EXPLORER trial (NCT02561988) and phase II PATHFINDER trial (NCT03580655), both of which investigated the efficacy and safety of avapritinib for treatment-naïve and pretreated patients with AdvSM.2,3
Andreas Reiter1 presented a pre-specified interim analysis for the PATHFINDER trial at the 26th European Hematology Association (EHA) Annual Congress. Key results that supported the decision for approval are summarized below.
Study design
The PATHFINDER study was a phase II, open-label, single-arm study in adult patients with AdvSM. The study design is summarized in Figure 1.
Figure 1. PATHFINDER study design*
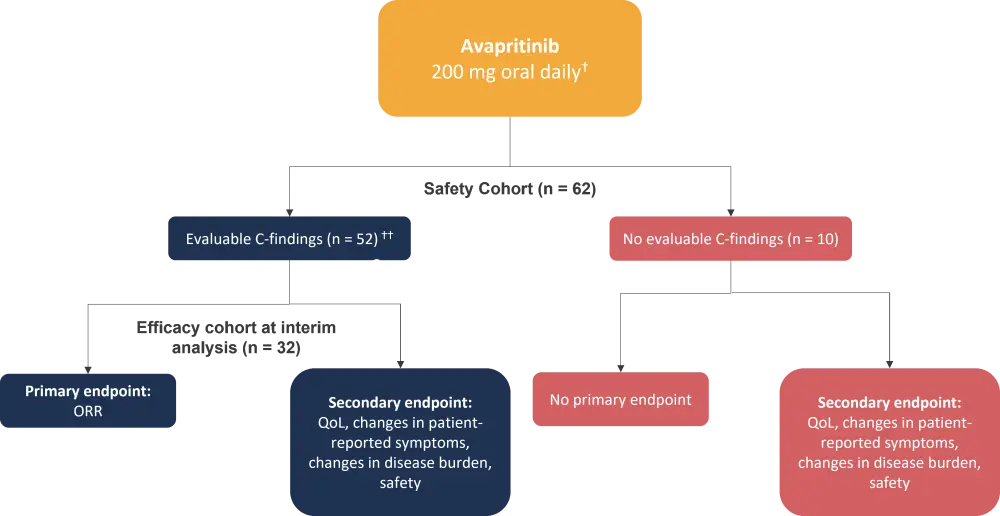
ORR, overall response rate; QoL; quality of life.
*Adapted from Reiter, et al.1
ⴕEvaluable C-findings: Absolute neutrophil count <1000/µL, hemoglobin value <10 g/µL, platelet count <100,000/µL, hepatomegaly with ascites and impaired liver function, palpable splenomegaly with hypersplenism.
†† Two patients were treated with 100 mg avapritinib oral daily.
Eligibility criteria:
- Central diagnosis of AdvSM
- ≥18 years of age
- Eastern Cooperative Oncology Group (ECOG) score of 0–3
- Patients with a platelet count <50 x 109 L excluded
Results
Patient characteristics for the safety (n = 62) and efficacy (n = 32) cohort are summarized in Table 1.
Table 1. Patient characteristics*
|
AdvSM, advanced systemic mastocytosis; ASM, aggressive systemic mastocytosis; BM, bone marrow; CMML, chronic myelomonocytic leukemia; ECOG, Eastern Cooperative Oncology Group, MC, mast cell; MCL, mast cell leukemia; MDS, myelodysplastic syndrome; MPN-U, myeloproliferative neoplasm unclassifiable; SM-AHN, systemic mastocytosis with associated hematologic neoplasm. |
||
|
Characteristic |
Safety population |
Efficacy population |
|---|---|---|
|
Median age, years (range) |
69 (31–88) |
68 (37–85) |
|
Female, % |
45 |
44 |
|
ECOG performance status 2–3, % |
31 |
34 |
|
AdvSM subtype, % |
||
|
ASM |
15 |
6 |
|
SM-AHN |
69 |
81 |
|
MCL |
16 |
13 |
|
KIT D816V mutation positive in blood, % |
95 |
94 |
|
Prior neo-plastic therapy, % |
||
|
Midostaurin |
55 |
53 |
|
Cladribine |
13 |
13 |
|
BM biopsy MC burden, median % (range) |
45 (1–95) |
50 (10–95) |
|
Serum tryptase level, median ng/ml (range) |
283 (24–1,600) |
293 (24–1,600) |
Primary endpoint
- The ORR for the efficacy cohort was 75% (n = 32).
- No significant difference was found between the AdvSM subtypes or prior treatment status (Table 2).
- Response was ongoing at the point of analysis (median follow-up, 10.4 months).
- Median time to response was rapid (2 months).
- Patient response improved over time, with a median time to complete remission with hematologic recovery of 5.6 months.
Table 2. Summary of response to avapritinib across AdvSM subtypes and prior or no prior therapy*
|
ASM, aggressive systemic mastocytosis; CI, clinical improvement; CRh, complete remission with partial hematologic recovery; MCL, mast cell leukemia; NE, not evaluable; ORR, overall response rate; PD, progressive disease; PR, partial remission; SD, stable disease; SM-AHN, systemic mastocytosis with associated hematologic neoplasm. |
||||||
|
Response, % |
|
Subtypes |
Prior therapy |
|||
|---|---|---|---|---|---|---|
|
ASM |
SM-AHN |
MCL |
Yes |
No |
||
|
ORR |
75 |
100 |
81 |
25 |
74 |
78 |
|
CRh |
19 |
50 |
19 |
0 |
13 |
33 |
|
PR |
31 |
50 |
31 |
25 |
30 |
33 |
|
CI |
25 |
0 |
31 |
0 |
30 |
11 |
|
SD |
13 |
0 |
8 |
50 |
9 |
22 |
|
PD |
3 |
0 |
0 |
25 |
4 |
0 |
|
NE |
9 |
0 |
12 |
0 |
13 |
0 |
Molecular response
- The authors observed a ≥50% reduction in the number of mast cells in 88% of patients, while 60% of patients achieved complete elimination of marrow mast cell aggregates.
- 93% of patients achieved ≥50% reduction in serum tryptase, while 43% achieved reduction to <20 ng/ml.
- Treatment with avapritinib led to a significant reduction in KIT D816V mutation allele frequency in the blood for patients with SM-AHN, indicating a reduction in KIT D816V clonal activity outside of mast cells, e.g., AHN component.
- 80% of this cohort achieved a ≥50% reduction in absolute monocyte count, while 88% achieved a ≥50% reduction in absolute eosinophil counts.
Secondary endpoints
- Using the advanced mastocytosis symptom assessment form (AdvSM-SAF) to classify patient-reported symptoms, Reiter reported a significant reduction both in total symptom score and in individual symptom scores, including fatigue, abdominal pain, and flushing.
Safety
When assessing the safety population (n = 62), 84% of patients remained on treatment while 5% discontinued treatment due to adverse events (AEs). No treatment-related deaths were reported. A summary of AEs is shown in Table 3.
Table 3. AEs reported in ≥ 15% of patients*
|
AE, adverse event |
|
|
AEs in ≥15% of patients |
Grade 3/4 |
|---|---|
|
Non-hematologic, % |
|
|
Peripheral edema |
3 |
|
Periorbital edema |
3 |
|
Diarrhea |
2 |
|
Nausea |
2 |
|
Vomiting |
2 |
|
Fatigue |
3 |
|
Hematologic, % |
|
|
Thrombocytopenia |
16 |
|
Anemia |
16 |
|
Neutropenia |
24 |
- 68% of patients had dose reductions resulting from AEs, most commonly resulting from neutropenia (19%) and thrombocytopenia (18%).
- Only one case of subdural hematoma was reported, which was due to pre-existing severe thrombocytopenia.
- Subsequently, any patients with baseline platelet counts <50 x 109 L were excluded from avapritinib treatment.
Conclusion
The PATHFINDER interim analysis demonstrated promising efficacy and safety outcomes with avapritinib treatment across all subtypes of advanced systemic mastocytosis, irrespective of pre-treatment status. A high overall response rate (75%) was reported, which appears to be durable with responses ongoing. Using molecular response to gauge efficacy, avapritinib reduced mast cell burden for all subtypes, and for SM-AHN, reduced the burden of KIT D816V mutations in the blood. Finally, the safety profile was favorable, with no treatment-related deaths, few discontinuations, and low incidence of intracranial bleeding.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

