All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Best practice recommendations for the management of MPN in pregnant patients
Do you know... In the absence of any clear contraindications, what is the standard dose of aspirin recommended for patients with MPN during pregnancy?
The management of myeloproliferative neoplasms (MPN) in pregnant patients generates a distinctive set of patient and fetal challenges.1 Pregnancy and MPN generate a heightened risk of thrombosis and increased bleeding diathesis at the time of delivery and postpartum.1 Several fetal consequences also have a greater chance of occurring, including intra-uterine growth retardation, preeclampsia, placental insufficiency, stillbirth, and premature delivery.1,2 As a result, there are complex management strategies aimed at ensuring the best possible patient and fetal outcomes.
Here, we summarize two publications presenting guidance on MPN and pregnancy: “Myeloproliferative neoplasms and pregnancy: Overview and practice recommendations” by Naseema Gangat and Ayalew Tefferi, and “How we manage Philadelphia-negative myeloproliferative neoplasms in pregnancy” by Susan E. Robinson and Claire N. Harrison.
Overview of thrombotic risk1
Patients with essential thrombocytopenia (ET) and polycythemia vera (PV) experience an increased risk of both arterial and venous thrombotic events. Current thrombotic risk stratification models have limited applicability in determining pregnancy complications. The risk of venous thromboembolism (VTE) is increased 4- to 6-fold during pregnancy, and is at its greatest postpartum. This is due to pregnancy-induced hypercoagulability brought about by an increase in coagulation factor levels, impaired fibrinolysis due to a rise in plasminogen activator inhibitor-1 levels, and anatomical compression of the inferior vena cava and pelvic veins/left iliac veins inducing venous stasis. Accordingly, a meta-analysis of 756 pregnant patients with ET revealed a 1.3% incidence of VTE during pregnancy, and among 575 pregnancies with a postpartum follow-up, the rate of postpartum VTE was 1.8%.
Pre-pregnancy assessment2
Patients diagnosed with MPN and of a reproductive age should begin with a risk assessment according to disease status, concomitant illnesses, and prior pregnancy outcomes, followed by a discussion of risks and benefits of therapeutic options in pregnancy. These include aspirin, low-molecular-weight heparin, venesection, and cytoreductive agents. Criteria for the assessment of poor pregnancy outcomes and placental insufficiency in patients with MPN in the UK are shown in Figure 1.
Figure 1. UK classifications for poor pregnancy outcome and placental insufficiency in patients diagnosed with MPN*
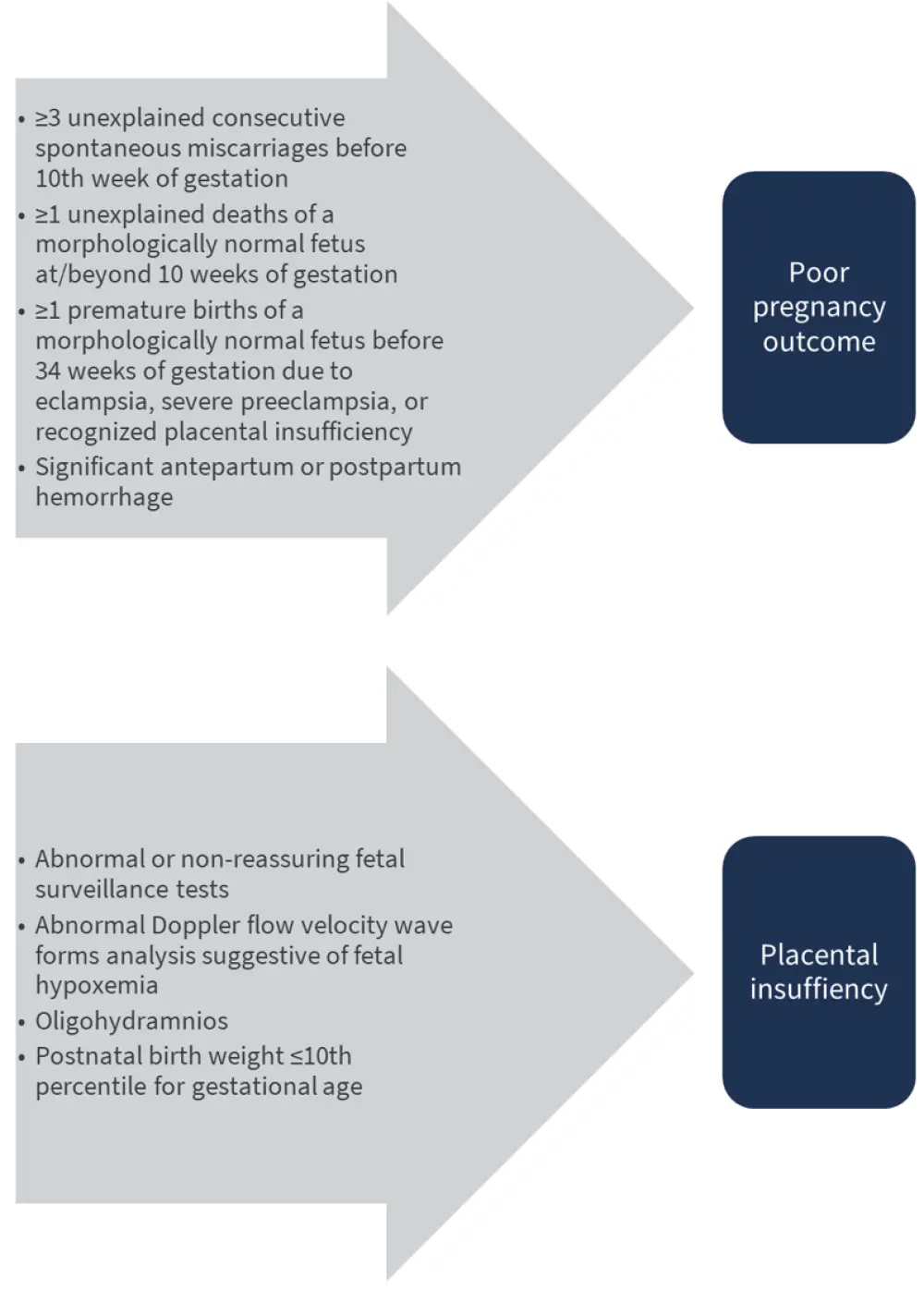
*Adapted from Robinson and Harrison. 2020.2
Management of MPN with pregnancy2
From preconceptual planning through postpartum phase, pregnant patients with MPN should have access to joint care in a multidisciplinary setting from a hematologist and an obstetrician with experience of high-risk pregnancies. Figure 2 depicts the UK guidelines for the management of pregnancy and MPN. Table 1 provides a summary of prophylactic approaches for pregnant patients with MPN in the UK.
Figure 2. UK guidelines for preconception to postpartum management for patients with MPN*
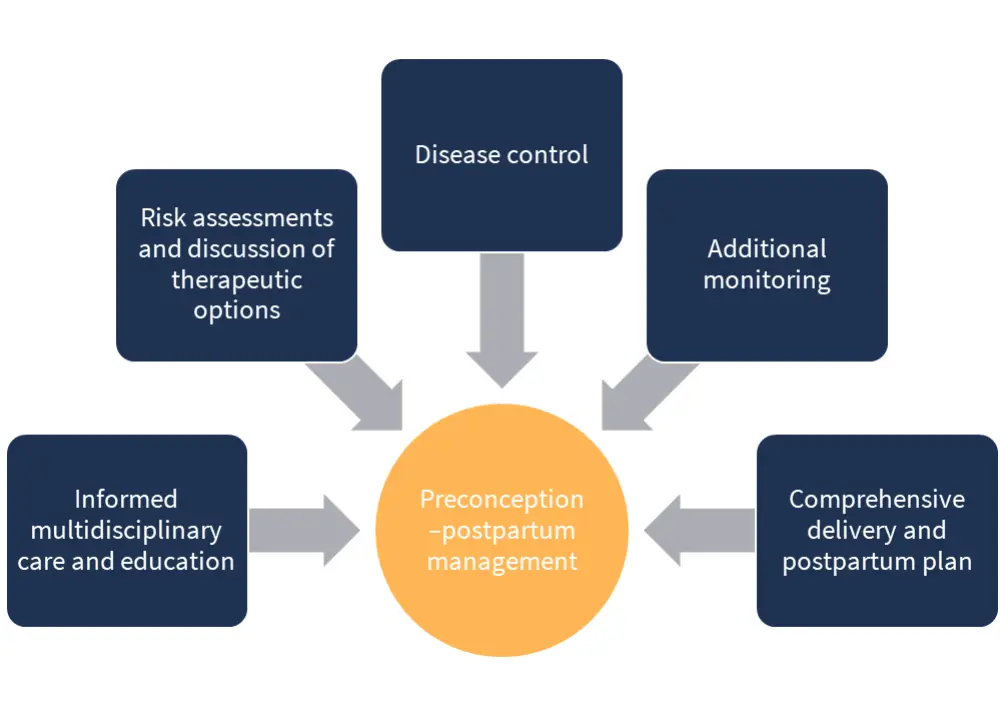
*Adapted from Robinson and Harrison. 2020.2
These guidelines help with optimal disease control and aim to increase the possibility of conception, implantation, and maintenance of placental function, thereby reducing complications resulting from placental dysfunction.
Aspirin2
Low-dose aspirin is considered safe in pregnancy. The Collaborative Low-dose Aspirin Study in Pregnancy (CLASP) Collaborative Group analyzed 32 randomized controlled trials including nearly 30,000 women; treatment with low-dose aspirin resulted in a 15% decrease in preeclampsia and a similar reduction in fetal death. Moreover, a recent meta-analysis reported live birth rates were higher in MPN pregnancies in patients who received low-dose aspirin compared with patients who were managed with observation alone. In addition, in patients with ET who had recurrent miscarriages, successful pregnancies have been recorded when treated with aspirin; however, some large studies do not support a positive effect of aspirin in this setting.
It is important to note that the use of low-dose aspirin must be considered in the presence of acquired von Willebrand syndrome (AVWS) with regard to a potential increased risk of bleeding. A review of 24 pregnancies in 18 women with ET found that AVWS-related abnormalities mostly improved during pregnancy, and no significant bleeding events during pregnancy or delivery were recorded. It was recommended that Von Willebrand factor parameters should be tested at early pregnancy and repeated at the third trimester, to guide pregnancy and delivery management.
In absence of clear contraindications, UK practice is to offer all patients 75 mg of aspirin once daily. If prior pregnancies have been associated with preterm eclampsia, the dosage should be increased to 150 mg once daily.
Low-molecular-weight heparin2
Prophylaxis with low-molecular-weight heparin (LMWH) is generally accepted when the absolute risk of VTE during pregnancy or the postpartum period is >3%. This is based on a net clinical benefit of preventing VTE that outweighs any harm as a result of major bleeding. In comparison, absolute VTE risk that is <1% does not warrant LMWH.
LMWH should be considered on the basis of additional risk factors as well as a preference and value-based discussion, given a modest absolute risk of VTE. The Royal College of Obstetricians and Gynaecologists recommends antenatal LMWH thromboprophylaxis in the presence of prior thrombosis or in the presence of one additional risk factor. In women with ET, the use of LMWH, based on an absolute risk of VTE, during the postpartum period to prevent thrombosis is suggested; all women are offered 6 weeks’ postpartum thromboprophylaxis with LMWH. These recommendations do not coincide with treatment recommendations for aspirin, which should be continued simultaneously.
Venesection2
During pregnancy, an increase in the plasma volume reduces the packed cell volume, altering red blood cell rheology. This phenomenon is important when reviewing optimal monitoring intervals and treatment targets. The target hematocrit for pregnant patients with MPN has generally been agreed to be <0.45 and in the mid-gestation-appropriate range. Furthermore, patients with PV and iron depletion are advised against taking iron replacement medication unless instructed by a hematologist.
Local practice in the UK involves isovolemic venesection to maintain the hematocrit levels in the required range:
- First trimester, 0.31–0.41
- Second trimester, 0.30–0.38
- Third trimester, 0.28–0.39
Cytoreductive therapy2
None of the cytoreductive drugs that are used to treat MPN have a license for use during pregnancy; however, if cytoreduction is considered imperative, the preferred therapeutic is interferon alfa. In the small numbers of pregnancies that have been exposed to interferon alfa, there have been no reports of teratogenic effects in animals or adverse effects. In a literature summary of over 90 pregnancies treated with interferon alfa, the live birth rate was 94%, thrombosis 1.3%, and major bleeding events 2.6%. Several forms of interferon alfa are currently in use; however, the pegylated form has become the agent of choice, and reasons for prescribing it to pregnant patients are as follows:
- If patients have an indication for cytoreductive therapy predating pregnancy
- If it is necessary to reduce platelet count
- If raised packed-cell volume is resistant to venesection
With regards to hydroxycarbamide, at least one case of fetal abnormality as well as teratogenicity in animal models has been reported; therefore, hydroxycarbamide is not recommended during conception and pregnancy. Anagrelide is also not advised due to its potential to cross the placenta and cause fetal thrombocytopenia.
JAK2 inhibitors2
JAK inhibitors are contraindicated in pregnancy, and there are no reports of pregnancy in women receiving JAK2 inhibitors. Routine use of a JAK inhibitor is not recommended.
Table 1. A summary of prophylactic approaches for pregnant patients with MPN in the UK*
|
AVWS, acquired von Willebrand syndrome; JAK2i, JAK2 inhibitors; LMWH, low-molecular-weight heparin; PV, polycythemia vera; VTE, venous thromboembolism. |
||||
|
Prophylaxis |
||||
|---|---|---|---|---|
|
Aspirin |
LMWH |
Venesection |
Cytoreductive therapy |
JAK2i |
|
Considered safe in pregnancy |
Generally accepted when absolute risk of VTE is >3% |
Not advised for patients with PV and iron depletion |
Not licensed for use during pregnancy |
Contraindicated in pregnancy—their use is not recommended |
Surveillance2
Uterine artery Doppler is a common predictive test for pregnancy complications, conducted between 18 and 24 weeks. A systematic review and meta-analysis revealed an increased pulsatility index (PI) to be the best predictor of preeclampsia, as well as overall and severe intrauterine growth restriction among low-risk patients. A positive result is a mean uterine artery PI >1.4, when escalating therapy and enhanced screening should be considered. UK practice is to offer full blood count monitoring, blood pressure, and urine testing every 4–6 weeks until 24 weeks; thereafter, at 2–4-week intervals. Pregnant patients with MPN are also offered uterine artery Dopplers to screen for placental dysfunction (mean PI >1.4 being a predictor of placental dysfunction) and serial growth scans during their pregnancy.
Delivery2
Prior to the time of delivery, it is important to discuss the implications of thromboprophylaxis for epidural or spinal anesthesia. If patients develop hemorrhagic problems while taking LMWH, treatment should be ceased and hematologic advice sought. In the UK, local practice birth guidelines for LMWH thromboprophylaxis antenatally include the following:
- Standard dose of LMWH thromboprophylaxis once daily
- If spontaneous labor, discontinue LMWH and present for assessment
- At least 12 hours between last LMWH dose before block performance or epidural catheter removal
- An additional 4 hours is required before restarting LMWH prophylaxis after block performance or catheter removal
Excess blood loss and blood transfusion are further risk factors for VTE; therefore, thromboprophylaxis should be begun or reinstituted as the immediate risk of hemorrhage is reduced. Aspirin should be considered throughout pregnancy unless there is evidence of a bleeding phenotype.
Postpartum2
During the postpartum phase, platelet counts and hematocrit levels may rise dramatically; however, these can be controlled through cytoreductive therapy or venesection followed by a blood count check at 6 weeks. The majority of patients receive LMWH, and oral iron is only given sparingly and at low doses for patients with PV.
Breastfeeding2
Aspirin has been found to be excreted in low concentrations in breast milk. This theoretically has the potential to cause platelet dysfunction and Reye’s syndrome due to immature metabolism and accumulation in the infants’ serum. There has, however, only been one report of infant toxicity, and studies have shown that acetylsalicylic acid transfer into breast milk is mostly undetectable. UK practice is to support breastfeeding while maintaining low-dose aspirin.
Heparins are not excreted in breast milk and may be used when breastfeeding; however, hydroxycarbamide, interferon alfa, and possibly anagrelide are excreted in breast milk so their use while breastfeeding is contraindicated.
Pregnancy outcomes in PV and PMF1
Recorded pregnancies are rare in patients with PV and PMF as patients are typically older (>60 years) at diagnosis. PV has a male predominance, with only 15% of patients diagnosed under the age of 40. Nevertheless, the European LeukemiaNet analyzed 121 pregnancies in 48 women, 39 of which were pre-PV diagnosis and 82 post-PV diagnosis. The live birth rate was 49% prior to diagnosis, as opposed to 77% post diagnosis; the majority of the latter patients were on aspirin and LMWH for 6 weeks postpartum in addition to phlebotomies for strict hematocrit control <40%, mainly in the first trimester. Twelve patients received interferon treatment, which was associated with a higher live birth rate of 83%; maternal thrombotic events were similar at 6% and 8% in pregnancies pre- and post-PV diagnosis, respectively; however, the incidence of bleeding events was higher in the latter group.
Pregnancy and primary myelofibrosis (PMF) are rare, and this is reflected by the scarce published literature. In a UK study, a total of four pregnancies in two women diagnosed with PMF recorded fetal loss in half the pregnancies, despite not receiving cytoreductive therapy. In contrast, a prospective study, also based in the UK, found that five patients diagnosed with PMF from a cohort of 58 patients with MPN all had live births.
Pregnancy outcomes in ET1
ET is the most prevalent MPN in young women; as such, pregnancy in patients with ET has been well described. The only prospective observational study of pregnancies in MPN was conducted in the UK between 2010 and 2012, describing a total of 58 pregnancies with MPN, of which 47 pregnancies occurred with ET. The study recorded 58 live births and one miscarriage and stillbirth each, which translated to an incidence of MPN pregnancies of 3.2/100,000 maternities per year, with an exceedingly low miscarriage rate at 1·7/100 pregnancies and perinatal mortality rate of 17/1,000.
In retrospective analyses, live birth rates in ET have varied from 41–90%, with spontaneous first trimester miscarriages at 25–50%. Predictors of fetal loss in ET are conflicting, with several studies showing no impact of driver mutations, whereas others demonstrate an increased loss in the presence of the JAK2V617F mutation. The role of aspirin, LMWH, and interferon alfa in preventing fetal loss remains unresolved.
A large retrospective study across 11 institutions based in Italy recorded 237 pregnancies in 158 patients with ET, and found 28.7% experienced fetal loss, with 88% occurring in the first trimester, together with a low rate of complications at 7%. The presence of the JAK2 mutation did not appear to impact the outcome of the pregnancy. Patients with the wild-type allele had a fetal loss rate of 27% compared with 35% in those with the mutant allele. Fetal loss was also comparable in patients with or without a history of thrombosis. Whilst thrombotic events before pregnancy had no impact on fetal or maternal outcomes, fetal loss was an independent factor of future maternal thrombotic events. At long-term follow-up, 18 patients had experienced major venous thrombosis postpartum, with future events more frequently occurring in patients with complications during pregnancy (28%) compared with patients without (3%).
Impact of driver mutations1
A cohort of 155 pregnancies in 94 patients with ET evaluated the impact of driver mutations (JAK2/CALR/MPL) on pregnancy outcomes and identified higher rates of late fetal loss with the JAK2 mutation, and a trend towards a lower incidence of pregnancy complications with presence of the CALR mutation. Complications were recorded in half of the pregnancies, with 30% experiencing fetal loss, 8.6% Intrauterine growth restriction, and 12% maternal events. Notably, 62.8% were JAK2-, 20% CALR-, and 2% MPL-mutated, and none of these mutations predicted overall fetal loss or complications. However, when second and third trimester losses only were evaluated, a higher rate of late fetal loss was recorded with JAK2V617F mutations compared with none in patients with MPL or CALR mutations and those who were triple-negative (P = 0.027). Furthermore, the presence of the CALR mutation resulted in a lower rate of pregnancy complications (P = 0.060).
Conclusion
While the combination of MPN and pregnancy is often a rare event, it is not without risks to both mother and fetus. Robust forms of care and management are in place that allow for most pregnancies to be successful; however risks need to be strictly controlled and there is always scope for advancement and refinement of management.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

