All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Chronic neutrophilic leukemia: A 2022 update on this rare disease and its management
Do you know... A 71-year-old patient has been diagnosed with CNL with an isolated CSF3R truncation mutation. They are symptomatic with bone pain and splenomegaly, and considered to be unsuitable for allo-HSCT. Which of the following statements is incorrect?
Chronic neutrophilic leukemia (CNL) is a very rare but often aggressive myeloproliferative neoplasm (MPN) characterized by mature neutrophilic leukocytosis, bone marrow granulocyte hyperplasia, and hepatosplenomegaly.1 A recent population-based study found an overall incidence of 0.1 cases/1,000,000 individuals.2 Given its rarity and the resulting lack of research, there is currently no established standard of care for the management of this condition.
Szuber and colleagues1 published their 2022 update on diagnosis, genomic landscape, prognosis, and management of CNL in the American Journal of Hematology. Their paper is summarized below, with a particular focus on the management strategies.
Disease overview
CNL tends to present in the older patient, with a median age at diagnosis of 66.5 years, and it shows a slight male predominance. More frequently it presents as incidental neutrophilic leukocytosis in an asymptomatic patient; however, it may also manifest with systemic symptoms such as fatigue, which is the most common, and bone pain.
Persistent chronic, mature neutrophilia is the hallmark of CNL, with a notable absence of monocytosis, basophilia, and eosinophilia. Patients may also present with mild anemia, thrombocytopenia, elevated lactate dehydrogenase, and elevated vitamin B12 levels.
The prognosis of CNL is variable. A median survival of 24 months was reported most recently, which is roughly in keeping with previous literature.3
Stages of CNL disease have not been formally classified, but its natural history seems to emulate that of untreated chronic myeloid leukemia (CML) with chronic, accelerated, and blast crisis phases. Disease progression presents by treatment resistance, progressive refractory neutrophilia, increase in transfusion dependency, worsening organomegaly, the occurrence of additional molecular or cytogenetic abnormalities, and blast crisis. Leukemic transformation, usually to acute myeloid leukemia (AML), occurs in 10–21.2% of cases with a median time to transformation of 21 months.
Molecular pathogenesis
The presence of activating CSF3R mutations in a high proportion of patients with CNL was hailed as a landmark discovery that revolutionized the diagnostic criteria for CNL, with the World Health Organization (WHO) revising the criteria accordingly in 2016 (see criteria below). In summary:
- Two mutational variants of CSF3R were discovered:
- membrane proximal mutations (mainly T618I and T615A point mutations), and
- frameshift or nonsense mutations leading to premature truncation of the protein.
- CSF3R T618I, the most common CSF3R mutation, has been shown to act via the JAK-STAT signaling pathway using a murine bone marrow transplant model (since responses were observed when using treatment with ruxolitinib, a JAK 1/2 inhibitor).
- Up to 30% of patients with CSF3R-mutated CNL harbor both membrane-proximal and truncation mutations.
- Patients with CSF3R-mutated CNL may also harbor concurrent mutations such as ASXL1, mutations in SETBP1, SRSF2, and less commonly, signaling mutations such as JAK2.
- Several studies have found that the presence of ASXL1 mutation is independently predictive of reduced survival.
Diagnosis
WHO 2016 revised diagnostic criteria for CNL:
- Peripheral blood leukocytosis ≥25 × 109/L
- segmented neutrophils plus band forms ≥80% of white blood cells (WBC)
- neutrophil precursors (promyelocytes, myelocytes, and metamyelocytes) <10% of WBC
- myeloblasts are rarely observed
- monocyte count is <1 × 109/L
- no dysgranulopoiesis
- Hypercellular bone marrow
- neutrophil granulocytes are increased in percentage and number
- normal neutrophil maturation
- myeloblasts <5% of nucleated cells
- WHO criteria for BCR-ABL1+ CML, polycythemia vera (PV), essential thrombocytopenia (ET), or primary myelofibrosis (PMF) are not met
- No rearrangement of PDGFRA, PDGFRB, FGFR1, or PCM1-JAK2
- Presence of CSF3R T618I (the most common mutation) or other activating CSF3R mutation, OR
- in the absence of a CSF3R mutation, persistent neutrophilia for ≥3 months, splenomegaly and no identifiable cause of reactive neutrophilia including absence of a plasma cell neoplasm or, if present, demonstration of clonality of myeloid cells by cytogenetic or molecular studies
Management
Szuber et al.1 propose a treatment algorithm based on current research available (see Figure 1). After diagnosis, as well as considering symptom burden, they propose mutation profile assessment and risk stratification to aid management decisions.
Using data from 19 patients with CSFR3-mutated CNL from the Mayo Clinic, Szuber et al.4 created a risk model predicting long-term survival. Based on platelet count, leukocyte count, and the presence of an ASXL1 mutation, patients can be classified as either low or high risk. The authors suggest that high-risk patients should undergo closer monitoring and considered earlier for allogeneic hematopoietic stem cell transplantation (allo-HSCT).
While clear evidence on when to initiate pharmacological treatment is lacking, Szuber et al.1 propose initiation of drug therapy at any timepoint for either uncontrolled myeloproliferation and/or associated symptoms. A leukocyte count <25–30 × 109/L is considered an appropriate target.
Figure 1. Treatment algorithm and risk stratification*
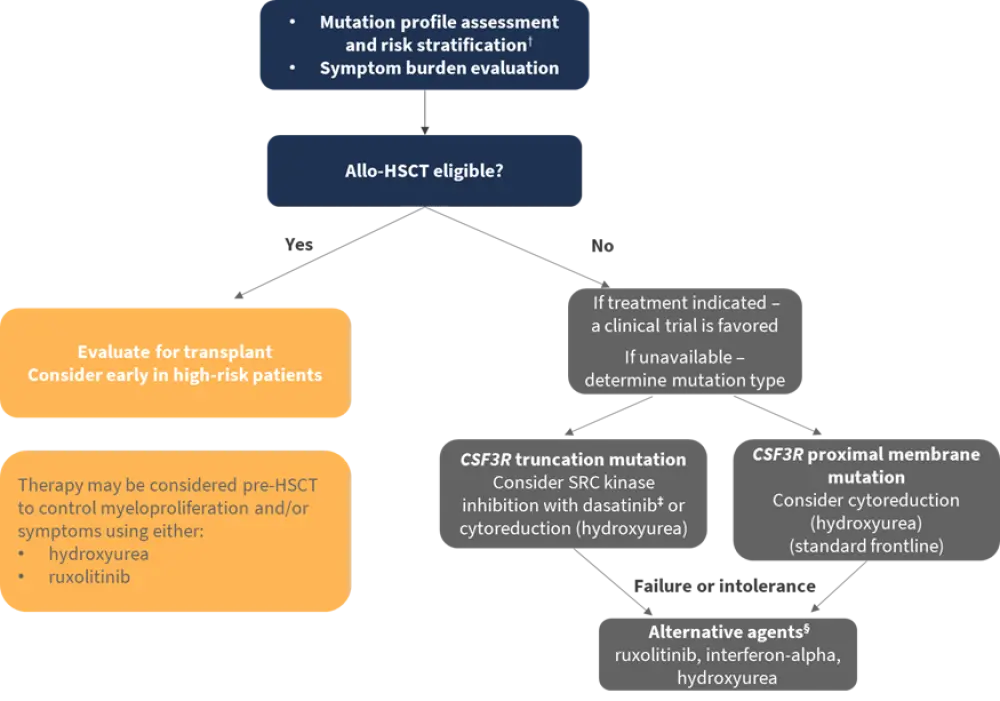
CSF3R, colony-stimulating factor 3 receptor; HSCT; hematopoietic stem cell transplantation.
*Adapted from Szuber et al.1
†Risk stratification: platelet <160 × 109/L (2 points); leukocyte count >60 × 109/L (1 point); ASXL1 mutation (1 point); low risk (0–1 point); high risk (2–4 points).
‡Caution, based primarily on in vitro data.
§Depending on frontline therapy.
Allo-HSCT
To date, allo-HSCT is the only therapy to meaningly improve survival in patients with CNL; however, this is limited to a minority of eligible patients and literature on allo-HSCT in patients with CNL remains sparse. Patients undergoing allo-HSCT in the chronic phase of CNL were found to have more durable remission compared to those who underwent allo-HSCT in the accelerated phase. Patients transplanted in the blast phase have worse outcomes with higher rates of early relapse and increased chemotherapy regimen-related toxicity. Additional studies are needed to define optimal timing, consider alternative stem cell sources, and myeloablative vs nonmyeloablative approaches.
Hydroxyurea and interferon-alpha
Oral chemotherapy agent hydroxyurea is the most commonly used first-line agent in clinical practice and up to 75% of patients show an initial response of reduced leukocytosis and/or reduction in splenomegaly with a 12-month median treatment duration. With only transient effects however, the majority of patients require second- and often third-line therapies (53% and 32% of patients, respectively, in the Mayo Clinic study).4 Other agents used in that study included interferon-alpha (IFN-a), hypomethylating agents (HMAs), ruxolitinib, thalidomide, cladribine, and imatinib.4
In limited case reports, IFN-a is the only agent that has shown potential for durable remission; thus, Szuber et al.1 recommend it as a safe and effective first-line agent in patients of childbearing age, or as a second/subsequent-line agent after the failure of previous therapy.
Ruxolitinib
The JAK1/2 inhibitor ruxolitinib is not U.S. Food and Drug Administration (FDA)-approved for CNL but given its ability to inhibit oncogenic JAK-STAT pathway signaling, it’s been proposed as a potential treatment. Study of its use in murine models and in patients with CNL harboring the CSF3R T618I mutation has yielded varied responses. Recently, a phase II prospective trial evaluating the safety and efficacy of ruxolitinib in patients with CNL (n = 21) found that the overall response rate was 35%, with four complete responses and nine partial responses.5 Clinical benefit in at least one category was seen in 85% of the cohort. A CNL diagnosis (vs atypical CML) and presence of the CSF3R mutation significantly correlated with ruxolitinib response. Notably, longer median survival times were seen in responders compared to nonresponders (23.1 vs 15.6 months).5 Effects of ruxolitinib on allele burden, and in turn its correlation to symptom improvement, remain indeterminate.
Concurrent mutations are speculated to contribute to the variability in clinical responses seen with ruxolitinib; however, study results have been inconsistent. CNL cases coexpressing CSF3R and SETBP1 mutations, a combination reported to carry a worse prognosis, were considered in several studies with conflicting results. Also, while compound CSF3R mutants in a murine model of CNL show ruxolitinib resistance, a recent case report found that ruxolitinib therapy induced a durable remission (3+ years) in a patient with CNL with compound CSF3R mutations following treatment failure with dasatinib.
Also of interest, is the potential for clonal evolution while on ruxolitinib. STAT3 mutations emerged late in ruxolitinib treatment, and it has been proposed that this may contribute to treatment resistance. Furthermore, RUNX1 and STAG2 mutations were detected at disease progression. As well as identifying possible late biomarkers of disease progression, the study highlights the potential need to target additional mutations in the efforts to overcome treatment resistance and eradicate malignant clones/subclones.
As yet, robust data on timing of ruxolitinib initiation, its place in the treatment sequence, and its role pre-allo-HSCT is lacking. Given its current use and study as primarily a second-line therapy, Szuber et al.1 propose that this is the most evidence-based approach and may be considered as an alternative in patients who are ineligible for allo-HSCT. Early data and extrapolation from its use in MPNs such as myelofibrosis in the peri-transplant period could justify its consideration in patients with CNL undergoing allo-HSCT.
Dasatinib
Dasatinib, an SRC kinase inhibitor, is proposed as a potential treatment option for patients with CNL with CSF3R truncation mutations, given that these mutations predominantly operate through SRC kinases, and have exhibited in vitro drug sensitivity. Research in patients with CNL is lacking; however, concern exists that with the additional proximal membrane mutations that often co-occur in CNL, dasatinib activity in CNL patients in a clinical setting would be insufficient. Szuber et al.1 propose it as potential treatment in this subset of CNL patients, with a note of caution given the lack of clinical evidence.
Other considerations
The standard ‘7+3’ induction chemotherapy regimen (daunorubicin and cytarabine) does not appear to induce hematologic remission in patients with CNL.
Also of note, while both splenic irradiation and splenectomy have been used to palliate symptomatic splenomegaly in patients with CNL, splenectomy is not recommended in CNL after postoperative reports of worsening neutrophilia.
Conclusion
CNL is a rare condition that presents a significant management challenge. Based on the limited literature available, Szuber et al.1 propose a treatment algorithm to guide management in the absence of clear and robust guidelines. Future prospective trials are required to better address key questions that remain unanswered, including the timing of allo-HSCT, initiation of drug therapy, and treatment sequencing.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

