All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Editorial theme | Pathogenesis and management of splanchnic vein thrombosis in MPN
Do you know... Which of the statements listed below regarding the pathogenesis of splanchnic vein thrombosis is false?
This editorial theme article will discuss splanchnic vein thrombosis (SVT) in myeloproliferative neoplasms (MPN), with a particular emphasis on pathogenesis, diagnosis, and clinical management of the condition.
In order to review this clinical area, two articles are summarized here; a review of the current clinical landscape of SVT (Tremblay, et al.1) and a review of SVT in relation to current anticoagulant therapies (Sedhom, et al.2).
Pathogenesis
SVTs present as venous thromboembolisms involving the portal, hepatic, splenic, and mesenteric veins.1 Patients diagnosed with MPN have a significantly higher risk of developing SVT compared with a healthy population, and this pathology is often driven by the commonly identified JAK2V617F mutation, which contributes to a procoagulant and pro-adhesive environment.1 In particular, patients with prior SVT have been shown to possess JAK2V617F mutant endothelial cells, which play a critical role in the increase in thrombotic risk (Figure 1).1
Figure 1. Effects of the JAK2 V617F mutation in endothelial cells*
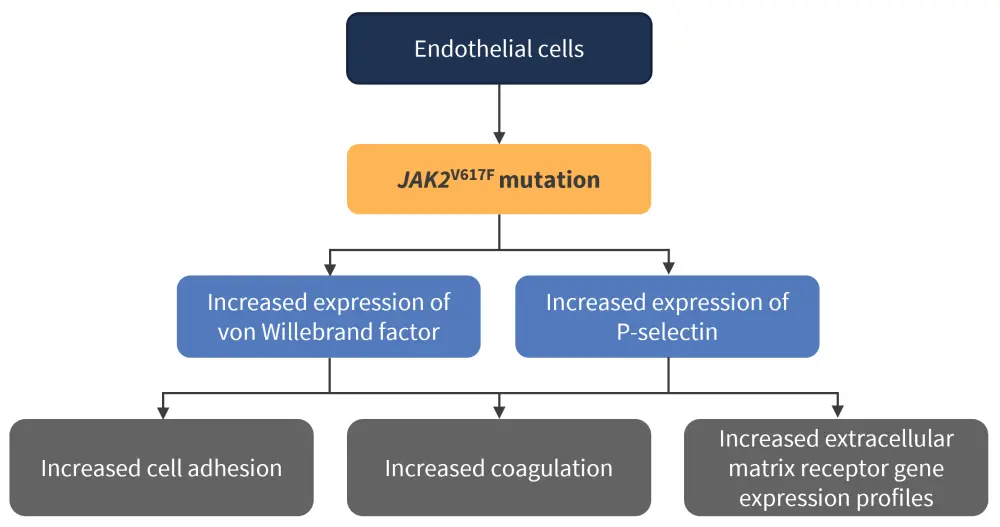
*Data from Tremblay, et al.1
In addition to the pathogenic role of JAK2-mutated endothelial cells, megakaryocytes with the JAK2 V617F mutation have demonstrated increased platelet signaling in response to thrombopoietin in mouse models and heightened chemotaxis leading to platelet aggregation in patients with MPN.2 Studies of molecular markers in relation to thrombosis revealed patients with JAK2V617F have increased levels of platelet, endothelial cell, and leukocyte activation compared with patients with wild-type JAK2.2
Within the splanchnic niche, recent in vitro data have suggested that shear caused by slow flow gradients contributes to prothrombotic gene expression.1 Together, the modified porto-systemic flow gradient combined with JAK2V617F mutation expression in liver sinusoidal endothelial cells lead to an increase in coagulation factor VIII and von Willebrand factor levels; and thus a higher thrombotic risk.1
The disruption of the nitric oxide signaling pathway is thought to be another important factor in the increased risk of SVT (Figure 2).1
Figure 2. Disruption of the nitric oxide signaling pathway and its effect on SVT risk*
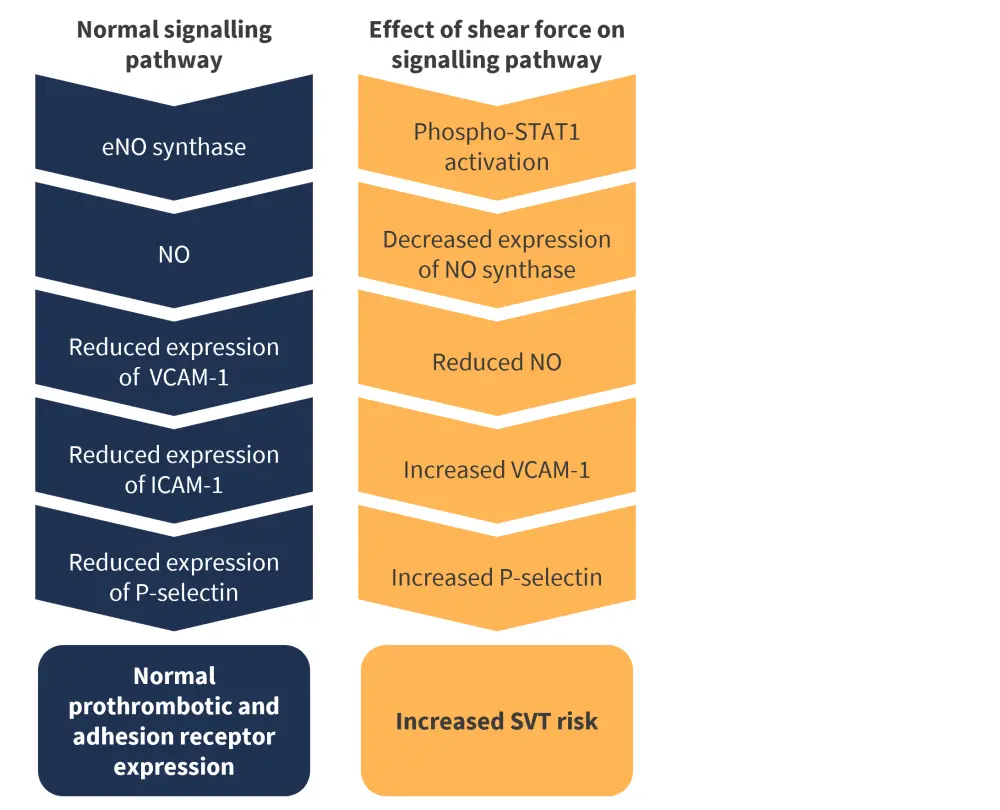
eNO, endothelial nitric oxide; ICAM-1, intracellular adhesion molecule 1; NO, nitric oxide; STAT1, signal transducer and activator of transcription 1; SVT, splanchnic vein thrombosis; VCAM-1, vascular cell adhesion molecule 1.
*Data from Tremblay, et al.1
Other signaling pathways that may contribute to increased SVT risk include the following:
- Low flow states in the splanchnic veins lead to hyperviscosity and contribute to altered hemostatic balance and increased thrombogenesis, by means of increased nitric oxide scavenging by erythrocyte hemoglobin.2
- Activation of STAT3 downstream of JAK2 increases cell adhesion molecule expression.1
- Endothelial cells produce plasminogen activator inhibitor-1, which binds to tissue plasminogen and urokinase plasminogen activator to decrease fibrinolysis.1
Epidemiology
SVT is most commonly diagnosed in patients <45 years, with over 70% of diagnoses in females.1,2 A high proportion of these patients present with a prior diagnosis of polycythemia vera and low allele burden JAK2V617F mutations.2 Polycythemia vera is also the most common MPN to present with SVT in all patients, followed by essential thrombocythemia, and then myelofibrosis.2 Other thrombophilic disorders have also been reported as independent risk factors for SVT.2
Diagnosis
Diagnosis of SVT is achieved with the following radiographic techniques1:
- Ultrasound by doppler imaging
- Computed tomographic scan
- Abdominal magnetic resonance imaging
The advantages and disadvantages of these imaging methods are shown in Figure 3.
Figure 3. Advantages and disadvantages of SVT imaging techniques*
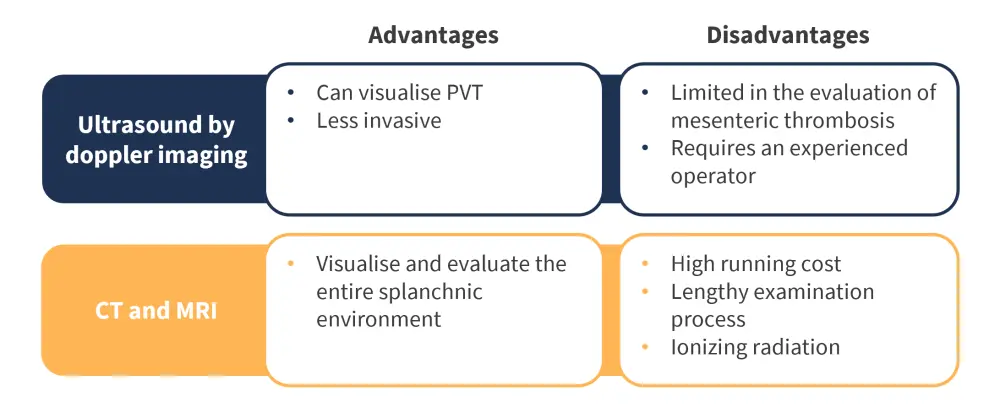
CT, computed tomographic scan; MRI, magnetic resonance imaging; PVT, portal vein thrombosis; SVT, splanchnic vein thrombosis.
*Data from Tremblay, et al.1
With regards to hematologic parameters, it is not uncommon for patients to present with normal blood counts; therefore, patients presenting with SVT should also be evaluated for MPN regardless of hematologic presentation.1 Extended next-generation sequencing panels are another useful diagnostic technique used to confirm the presence of myeloid-associated mutations, specifically JAK2, CALR and MPL.1 However, these remain expensive and are not widely available at present.1
For some patients, a recognizable clinical presentation indicative of MPN may not present for several months and sometimes years. In cases where clinical features are suggestive of MPN, or a driver mutation has already been identified, a bone marrow biopsy is useful to confirm the diagnosis.1 Additional thrombophilia testing is not usually necessary, as it is unlikely to impact future treatment decisions.1 However, it should be performed in certain young patients who present with an additional autoimmune disease.1
Prognosis
While prognosis for patients is largely dictated by features of MPN, patients with MPN and SVT have an average life expectancy 5 years less than those without SVT.1,2 The drivers for this reduction in life expectancy are2:
- liver failure;
- major bleeding; and
- non-skin related secondary cancer.
Another recent study highlighted that patients with MPN and SVT had a higher frequency of1:
- risk of bleeding;
- venous thrombosis;
- secondary malignancies; and
- deaths from hepatic disease complications.
Interestingly, these findings contrast with earlier studies, which suggested that the underlying MPN condition and mutational profile of the patient had a greater impact on life expectancy.2
Management
While there is currently limited data around optimal SVT management, interdisciplinary approaches and co-ordination are critical for the improvement of patient outcomes. Anticoagulation therapy remains the primary form of treatment for patients with SVT and has several therapeutic effects (Figure 4).1
Figure 4. Therapeutic effects of anticoagulation therapy*
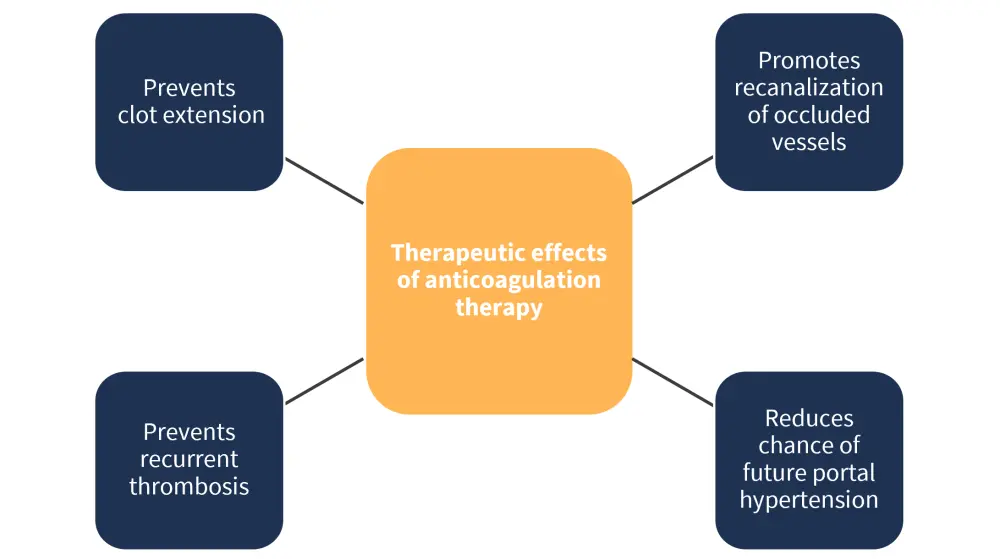
*Data from Tremblay, et al.1
Patients with portal hypertension at diagnosis may require a reduction in bleeding risk before anticoagulant therapy can begin.1 Cases of thrombocytopenia must also be carefully managed, whereby the benefits of treatment must outweigh the risks of bleeding.1 A full explanation of treatment considerations is shown in Figure 5.
Figure 5. Treatment considerations for SVT*
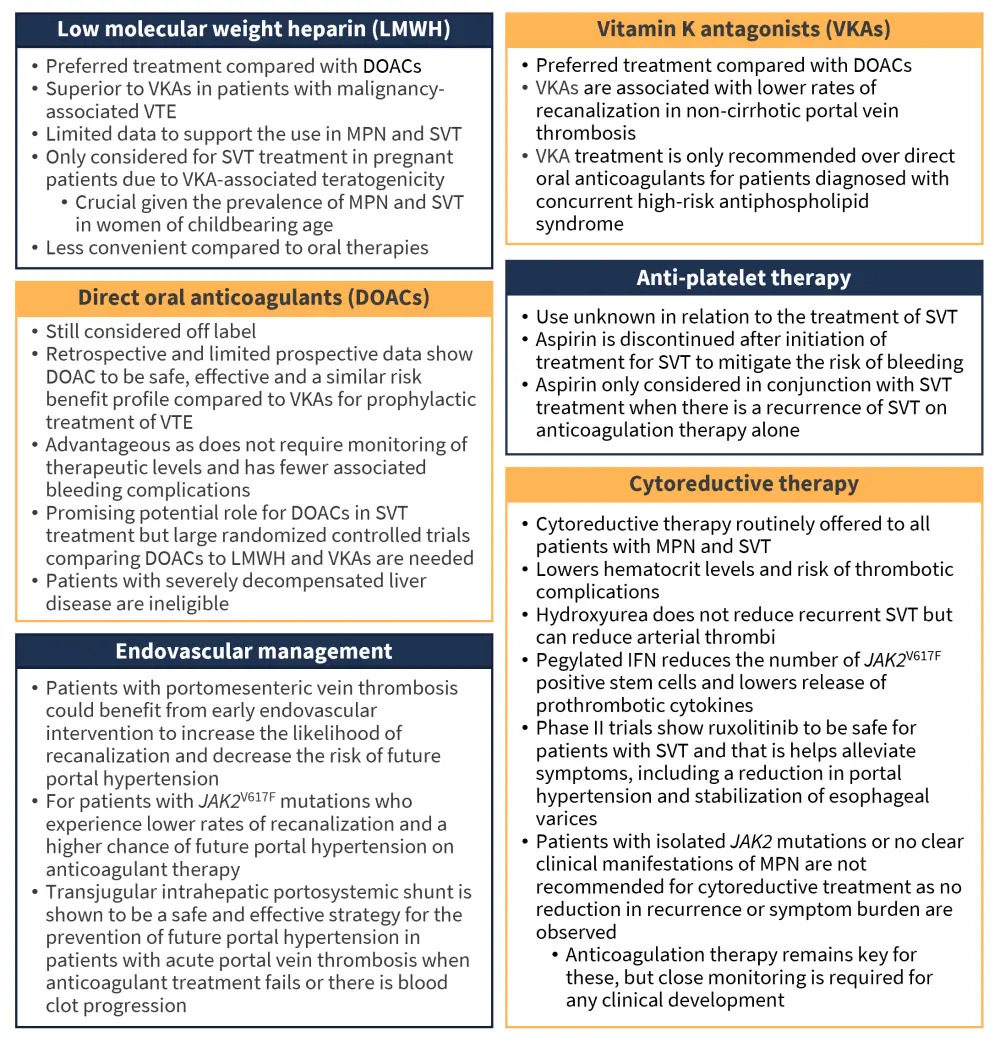
DOAC, direct oral anticoagulant; IFN, interferon; LMWH, low molecular weight heparin; MPN, myeloproliferative neoplasm; SVT, splanchnic vein thrombosis; VKA, vitamin K antagonist; VTE, venous thromboembolism.
*Data from Tremblay, et al.1 and Sedhom, et al.2
For most patients, anticoagulation therapy should be continued indefinitely due to the risk of recurrent thrombosis after the first SVT or upon treatment cessation.1 Should the risk of bleeding increase over time, the dosage can be reduced to prophylactic levels in order to avoid treatment discontinuation.1
The impact of SVT on allogeneic hematopoietic stem cell transplant outcomes has yet to be fully evaluated. One recent study showed that a history of SVT and portal hypertension lead to an increased risk of moderate-severe hyperbilirubinemia and hepatic sinusoidal obstruction syndrome posttransplant.1 While this is not a complete contraindicator for transplantation, necessary optimization and coordination are required in the presence of SVT in order to reduce any liver-associated morbidity.1
Conclusion
Younger female patients are most commonly diagnosed with MPN and SVT, which represents a reduced life expectancy compared with those diagnosed with MPN only. While anticoagulant therapy remains the primary form of managing SVT, the indefinite period of treatment requires regular re-evaluation to ensure the risk benefit profile remains balanced. Other forms of treatment including cytoreductive therapy and endovascular management have demonstrated promising signs of reducing the symptom burden and risks associated with SVT. However, larger clinical trials are still needed to confirm their effectiveness and overall role in clinical management.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

