All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
ELN 2021 guideline recommendations for polycythemia vera
The satisfactory management for patients with polycythemia vera (PV) has been elusive; particularly regarding patients under 60 years old without history of vascular events, who have low-risk disease, and who are treated with phlebotomies and low-dose aspirin, while still carrying a higher risk of vascular events than the general population.
The European LeukemiaNet (ELN) guideline evidence- and consensus-based recommendations for optimal cytoreductive treatment of PV were recently published in a review article by Marchetti et al. in The Lancet Haematology.1 We summarize these guidelines below.
Methodology
The process for creating the 2021 ELN guidelines was completed by an expert panel consisting of 14 senior hematologists from seven countries (Austria, France, Germany, Italy, Spain, UK, and US). The three main clinical questions, search criteria, and evidence quality grading are described in Figure 1.
Figure 1. Process for clinical recommendations based on three clinical questions in PV*
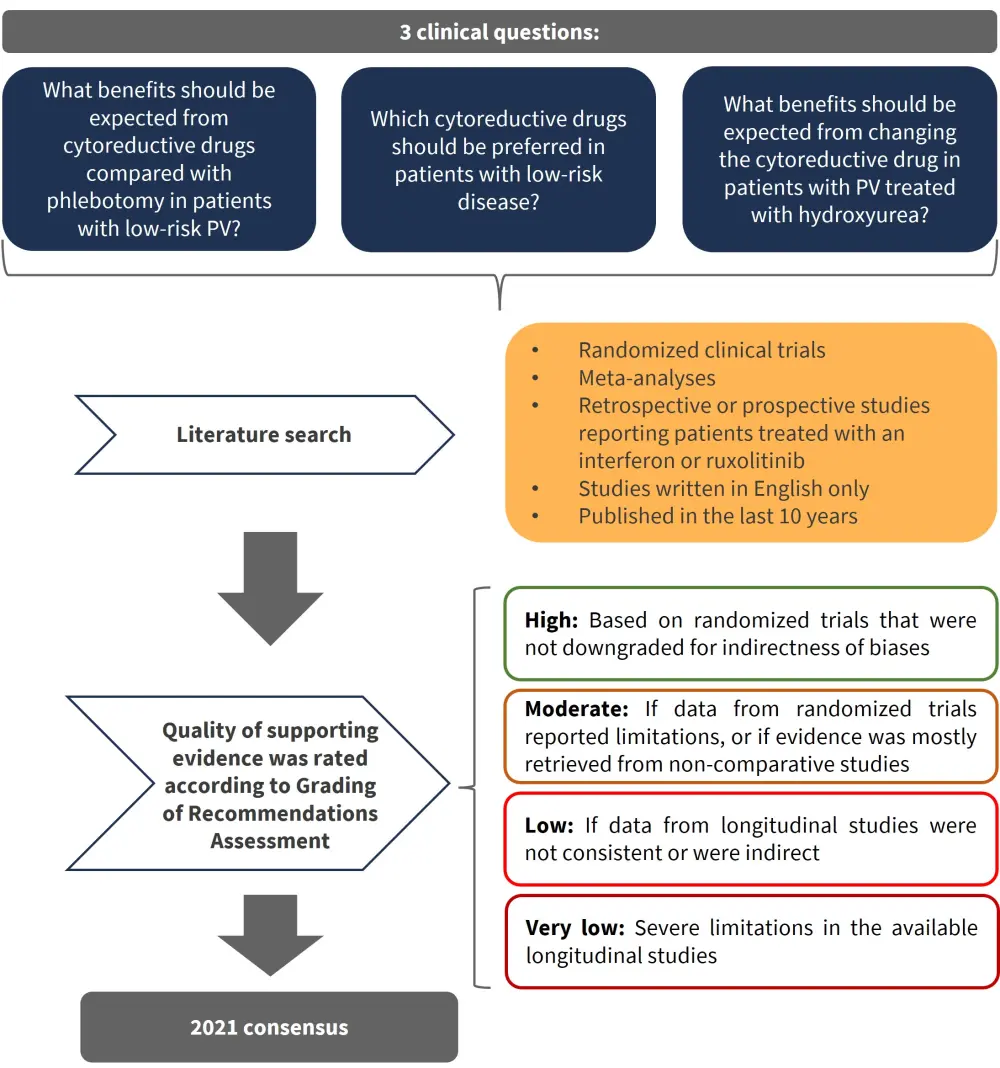
PV, polycythemia vera.
*Adapted from Marchetti et al.1
Results
The benefits expected from cytoreductive drugs over phlebotomy in low-risk PV
The question whether to use cytoreduce therapy over phlebotomy was based on three clinical outcomes:
- Reducing the risk of vascular events by normalizing blood counts and sustaining steady hematocrit levels
- Delaying disease transformation to myelofibrosis
- Improving disease-related symptoms
Appropriateness for cytoreductive treatment in patients with low-risk PV was governed by thrombotic risk which influenced PV-related symptoms, response to phlebotomy, and individual cardiovascular risk factors. Recommendations are summarized in Figure 2.
Figure 2. Clinical scenarios for which cytoreductive therapy is recommended in low-risk PV*

PV, polycythemia vera.
*Adapted from Marchetti et al.1
In patients with PV who are treatment-naïve, younger than 60 years, and without a history of vascular events but need cytoreductive therapy, the first choice should be ropeginterferon alfa-2b or pegylated interferon alfa-2a, unless contraindicated.
Which cytoreductive drugs should be preferred in low-risk patients?
The standard treatment for patients requiring cytoreductive therapy is hydroxyurea or interferon-alfa. The expert panel examined study evidence comparing these two treatment options. Extensive high-quality evidence indicated an improvement in hematocrit control, phlebotomy frequency, hematologic response, and molecular response when using interferon-alfa-2b over hydroxyurea. The panel’s recommendation was to
- use pegylated or non-pegylated interferon-alfa as cytoreductive therapy in patients younger than 60 years
- use hydroxyurea for those older than 60 years
As ropeginterferon alfa-2b is not available worldwide, the recommendations included any pegylated interferon-alfa formulations available.
Which patients with PV, treated with hydroxyurea, should receive a different cytoreductive drug?
Intolerance to hydroxyurea, secondary skin malignancies after hydroxyurea or ruxolitinib, or incomplete responses were previously reported by several studies and, therefore, the use of alternative cytoreductive approaches are encouraged; the clinical outcomes of patients with PV treated alternatively with ruxolitinib and interferon-alfa have been investigated.
The expert panel suggested that based on the evidence, either ruxolitinib or interferon-alfa could be used in patients with PV treated with hydroxyurea who require alternative cytoreductive therapy. Further details on these recommendations are shown in Figure 3.
Figure 3. Guideline recommendations for switching to ruxolitinib and interferon-alfa treatment*
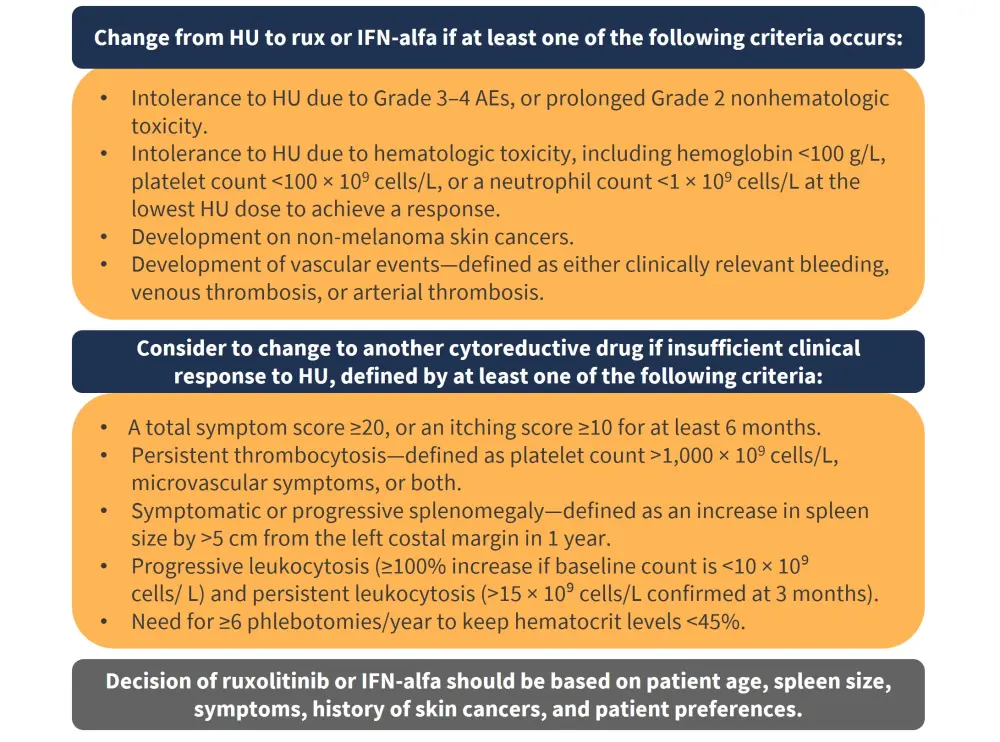
AEs, adverse events; HU, hydroxyurea; IFN-alfa, interferon-alfa; rux, ruxolitinib.
*Adapted from Marchetti et al.1
Conclusion
The ELN 2021 guidelines for PV have used a Grading of Recommendations Assessment, Development and Evaluation (GRADE)-based consensus to clarify recommendations based on three key clinical questions, and aimed to provide recommendations to prevent long-term undesirable outcomes while waiting for more robust data. In line with the 2018 ELN recommendations, patients aged >60 years should initiate therapy with either hydroxyurea or interferon-alfa. Patients with low-risk PV who have intolerance to phlebotomy, progressive splenomegaly, and persistent leukocytosis are recommended to receive cytoreductive therapy. Interferon-alfa is recommended over hydroxyurea for low-risk patients, and a change from hydroxyurea to either ruxolitinib or interferon-alfa is recommended in patients with intolerance to hydroxyurea due to Grade 3–4 or prolonged Grade 2 nonhematologic toxicity, or hematologic toxicity.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


