All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Factors affecting transplant outcomes in MF
Do you know... The DIPSS prognostic model is one of two models recommended for patient selection for HSCT when a patient presents without genetic information. Which is the second prognostic model, recently recommended in a review by Perram, et al., for risk assessment in patients without genetic information?
Allogeneic hematopoietic stem cell transplantation (HSCT) is considered the only curative therapy in patients with myelofibrosis (MF).1 Current guidelines provide recommendations for HSCT eligibility; however, the optimal patient selection or timing of HSCT remain unclear. In addition, nonrelapse mortality (NRM) is a concern, which is a challenge for improved outcomes in patients undergoing transplant.1
In response to this, Perram, et al.1 published a review on novel approaches to improve transplant outcomes in myelofibrosis in the American Journal of Hematology. We summarize the review below, looking at factors influencing NRM and considerations prior, during, and after transplant.
Factors associated with NRM
Secondary organ dysfunction may contribute to the high NRM observed in patients after transplantation and could be evaluated prior or during transplant.
Portal hypertension
Up to 18% of patients diagnosed with myeloproliferative neoplasms report portal hypertension arising from splanchnic thrombosis. Varices may increase the risk of significant bleeding during and after transplant. Therefore, screening for these events before transplant is recommended. Endoscopic screening can be considered for high-grade varices in case of increased risk of bleeding. For noncirrhotic portal hypertension, imaging techniques, such as Doppler ultrasound or magnetic resonance imaging (MRI) angiography, should be considered, as well as liver elastography, which also helps to exclude cirrhosis.
Impaired liver function
Portal hypertension, excessive iron levels, and splanchnic thrombosis may lead to hepatotoxicity during transplant, which in turn may lead to poorer survival. Liver iron levels should be evaluated via MRI screening before transplantation. In case of iron overload, a risk assessment should be performed to delay transplant and manage iron levels with chelation.
Pulmonary hypertension
Around half of patients with MF undergoing HSCT experience pulmonary hypertension as a result of cardiac risk factors and high levels of hematocrit or N-terminal pro-brain natriuretic peptide. Therefore, screening for natriuretic peptide and troponin levels and conducting an echocardiogram for estimating the pulmonary pressure before transplantation are advised. Invasive approaches, such as vasoreactivity, may be also needed to inform treatment decisions. If significant pulmonary hypertension is identified, a specialist cardiologist or respiratory physician should be consulted. These strategies would inform treatment decisions during or after transplantation if any related complications occur.
Extramedullary hematopoiesis
The effect of extramedullary hematopoiesis on NRM is currently unclear; however, the combination of tissue shrinkage after conditioning and thrombocytopenia may lead to non-typical bleeding complications due to the unstable nature of the tissue. While the effects remain unclear, patients are recommended to be screened with a form of radiologic imaging prior to transplantation. If there is a significant risk to the patient or sites of high risk are identified, targeted radiotherapy should be considered on an individual basis. However, pretransplant screening and low-dose irradiation are currently areas for further study.
Iron overload
Hepatic iron overload identified via MRI does not predict overall survival, NRM, relapse, or graft-versus-host disease for non-MF indications after HSCT. It has been suggested that reducing the toxic environment of the bone marrow by the addition of free iron may improve engraftment and the formation of blood cellular components. Patients who are at risk are recommended to undergo MRI screening to monitor iron overload of the heart and liver. Pretransplant chelation is recommended for patients with iron overload; however, it cannot be recommended as a routine delay and is therefore only appropriate in certain cases.
Graft function and failure
Poor graft function and failure present significant challenges for patients undergoing HSCT due to the poor ability of donor cells to survive in the marrow microenvironment. Increased levels of proinflammatory cytokines, tissue damage, and scaring, and often bone marrow fibrosis have all been reported to negatively affect donor cell proliferation. Blood pooling in the spleen, seen in some patients, has also been found to reduce stem cell localization. These challenges, in combination with a lack of comprehensive criteria for poor graft function and failure, present a considerable obstacle to improving patient outcomes. However, aligning clinical trial design with up-to-date consensus definitions would aid in the advancement of clinical outcomes. Unfortunately, the known risk factors for poor graft function and graft failure, including age, disease grade, and donor characteristics, are mostly unalterable. As a result, a significant adjustment is needed to improve management options, especially in cases where graft failure cannot be prevented.
Risk stratification models
Several risk stratification models have been developed to help in the selection of patients for HSCT; yet despite this, the rationale for transplantation remains challenging and the optimal disease and transplantation risk calculation for patients remains unclear. Therefore, diagnostic models that include molecular risk, such as the Genetically Inspired Prognostic Scoring System (GIPSS) or the Mutation-Enhanced International Prognostic Scoring System 70+ (MIPSS70+), are recommended. If the patient only has cytogenetic information available, then the MIPSS70+ or the Dynamic International Prognostic Scoring System+ (DIPSS+) are advised. Moreover, if genetic information is unavailable, then the DIPSS or MIPSS70 are recommended.
The clinical factors that potentially contribute to poor transplant outcomes are depicted in Figure 1.
Figure 1. Clinical factors that may lead to poor transplant outcomes*
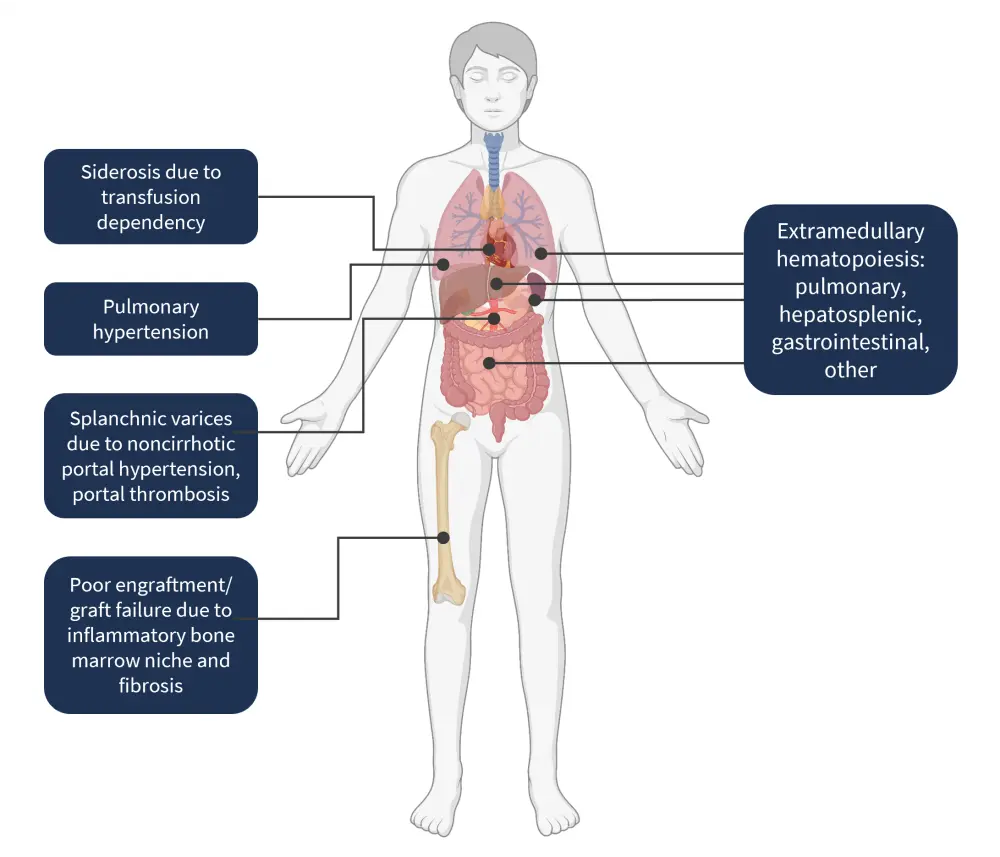
*Adapted from Perram, et al.1 Created with BioRender.com.
Factors to be considered throughout the transplant process
The process of HSCT encompasses multiple stages in order to give the best clinical outcome for the patient. Several factors must be addressed before, during, and after the transplantation process, which can be seen in detail in Figure 2.
Figure 2. Factors to be considered throughout the transplantation process*
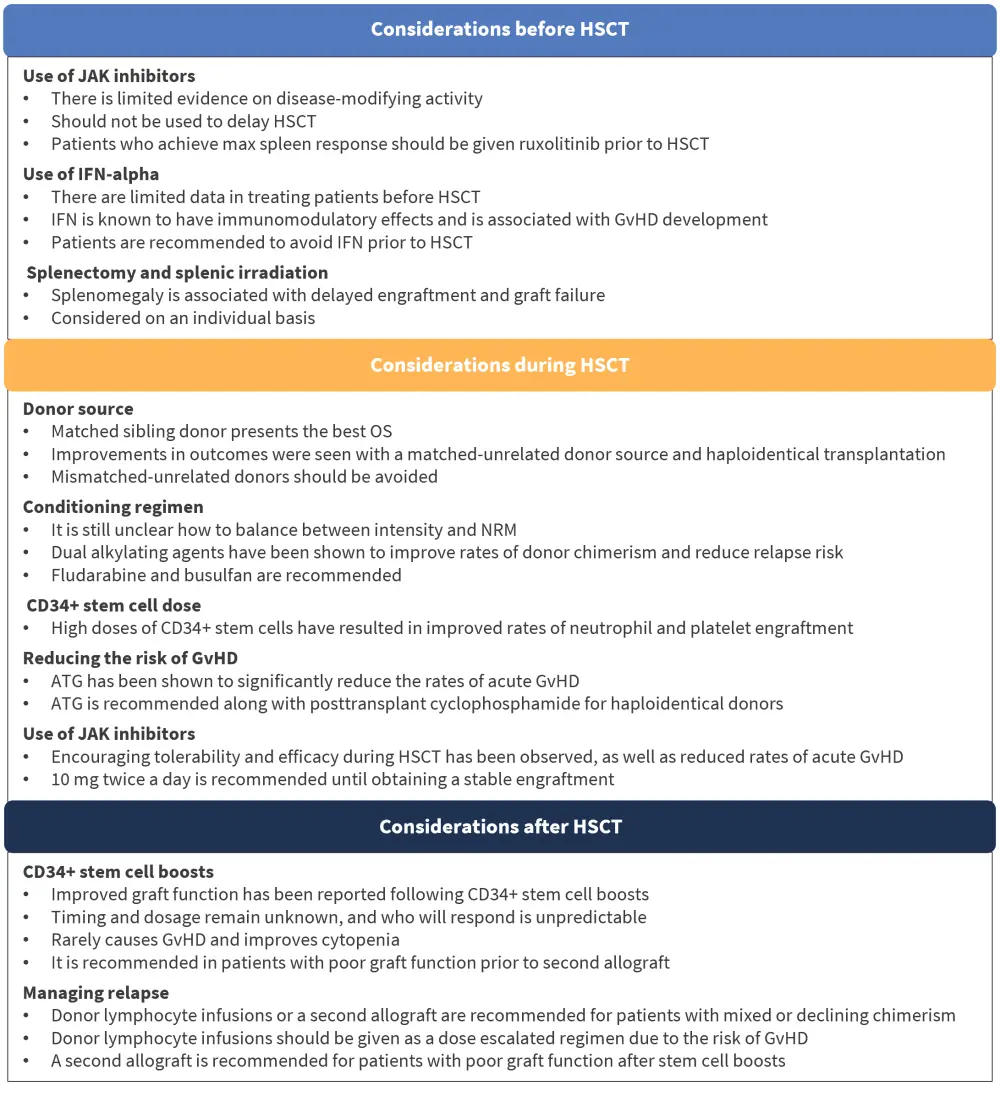
ATG, antithymocyte globulin; GvHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; IFN, interferon; NRM, nonrelapse mortality; OS, overall survival.
*Adapted from Perram, et al.1
Conclusion
Given the rarity of MF, international efforts are warranted to allow further research in this disease area. Transplant is considered the only curative option in patients with MF; however, there is a risk of transplant-associated morbidity and mortality, limiting the patient selection and outcomes. Disease-related factors (such as impaired organ function) and transplant-related factors (such as allografting) associated with poorer outcomes have been summarized above.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

