All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Impact of thrombosis on disease progression and mortality in polycythemia vera
Do you know... The progression to blast-phase disease is one of several diverse clinical characteristics experienced by patients with PV. What percentage of patients transitioned to blast-phase disease directly from a PV diagnosis?
Patients diagnosed with myeloproliferative neoplasia present with diverse clinical characteristics, each associated with varying risks of thrombosis, disease progression, and death. Previous risk analyses focusing on these characteristics have mostly relied on conventional statistical models, which do not fully account for the complexities of multiple time-varied outcomes. Furthermore, many conventional survival and hazard estimates do not consider the intermediate disease states and transitions that take place prior to the final state. Novel approaches, including multistate models, are needed for a more comprehensive understanding of risk and survival estimates.
To bridge this knowledge gap, Barbui et al.1 analyzed data from a large cohort of patients diagnosed with polycythemia vera (PV) using a multistate model, published in Blood Cancer Journal. The aim was to estimate the transitions between disease states and examine the impact of incident arterial and venous thrombosis on disease state occurrence and death. Below, we summarize the key points.
For further information on thrombotic and hemorrhagic complications in myeloproliferative neoplasms, watch this recent discussion from the MPN Hub Steering Committee meeting.
Study design1
- Re-analysis of the International Working Group for Myeloproliferative Neoplasms Research and Treatment cohort of 1,545 patients with PV
- A five-state parametric Markov survival model was used to describe the clinical course of PV
- Risk factors for progression and mortality were identified by a Cox regression model
Results1
Baseline patient characteristics at diagnosis are shown in Table 1.
Table 1. Baseline patient characteristics*
|
Hb, hemoglobin. |
|
|
Characteristic, % (unless otherwise specified) |
N = 1,545 |
|---|---|
|
Median age, years |
61 |
|
Male |
49 |
|
Female |
51 |
|
Median Hb, g/dL |
18.4 |
|
Median hematocrit |
55 |
|
Median leukocyte count, × 109/L |
10.4 |
|
Median platelet count, × 109/L |
466 |
|
Arterial thrombosis before/at diagnosis |
16 |
|
Venous thrombosis before/at diagnosis |
7 |
- The median follow-up was 6.9 years;
- during follow-up, 3% of patients transitioned to blast-phase disease, 9% progressed to post-PV myelofibrosis (MF), and 23% died; and
- The rate of thrombosis postdiagnosis was 2.62% per patient year, with 184 and 137 patients experiencing an arterial thrombotic event and a venous thrombotic event, respectively.
The causes of death according to disease state transition are shown in Figure 1.
Figure 1. Cause of death according to disease state transition*
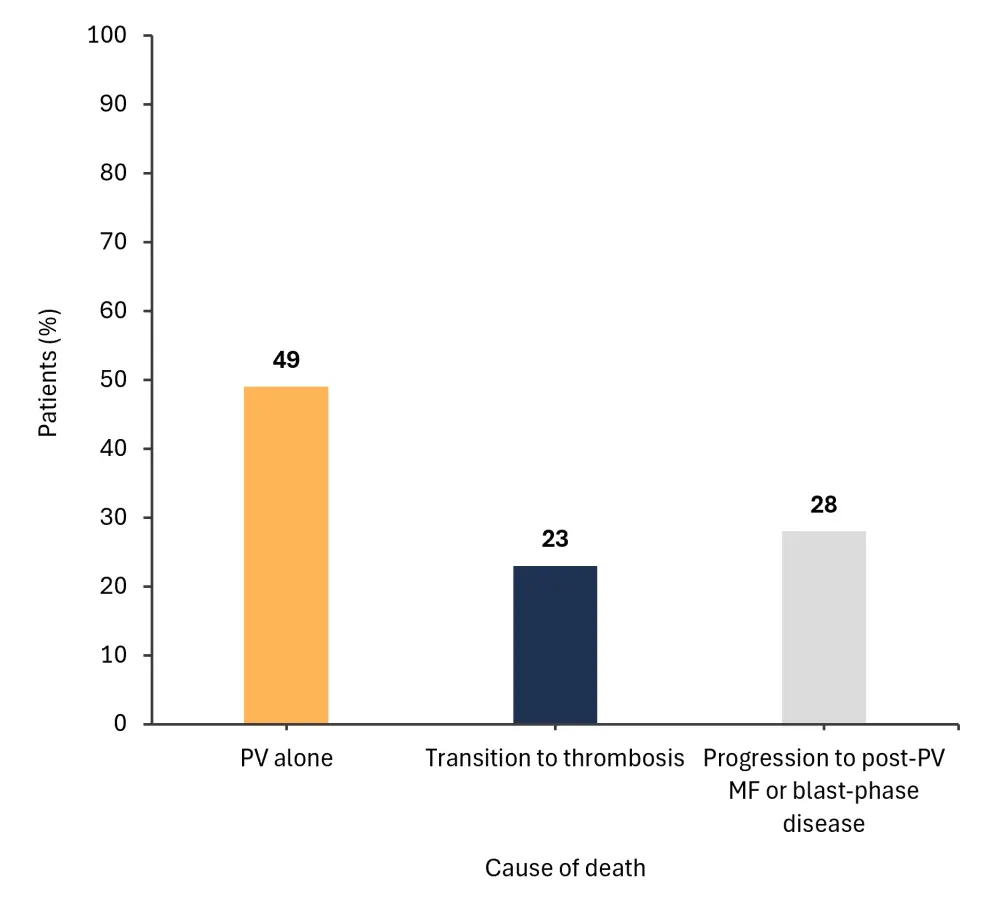
MF, myelofibrosis; PV, polycythemia vera.
*Adapted from Barbui, et al.1
- In total, 85% of patients experienced transition to post-PV MF directly from PV
- Transition from post-PV MF to blast-phase disease occurred in 32% of patients, compared with 44% who transitioned directly to blast-phase from PV
- A total of 282 patients experienced ≥1 major thrombotic event
The frequency of transitions to subsequent disease states from thrombosis is shown in Figure 2.
Figure 2. Frequency of transitions to subsequent disease states from thrombosis*
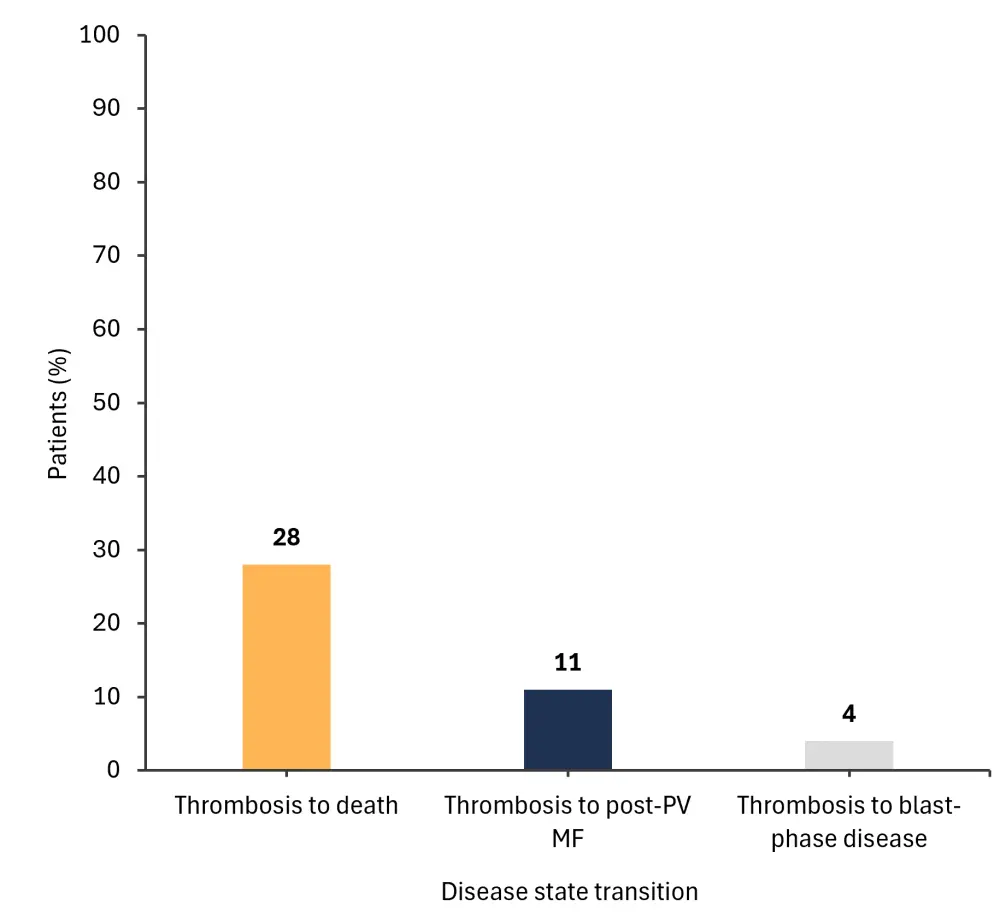
MF, myelofibrosis; PV, polycythemia vera.
*Adapted from Barbui, et al.1
- the probability of patients being event-free at 10 years postdiagnosis was 60%
- this reduced to 10% at 30 years
- the overall mortality rate was 40%
- the probability of progression to post-PV MF directly from PV diagnosis was progressive and linear over time
- the probability of thrombosis increased in the first 5 years postdiagnosis
- patients with thrombosis experienced an earlier acceleration of progression to blast-phase disease, compared with those who progressed from initial PV diagnosis only (2% vs 0.5% after 5 years)
- the accelerating effect of thrombosis tapered off after 10 years
- the probability of death in the first 10 years was higher in patients with thrombosis compared with those without (40% vs 20%; p < 0.01)
- patients >65 years old had a statistically higher risk of progression to blast-phase disease (p = 0.031)
- a history of venous thrombosis was also independently associated with an increased risk of death (p = 0.008)
- time-dependent analysis showed blast-phase disease was associated with venous thrombosis (p = 0.086)
- incident arterial thrombosis was also a significant risk factor for death (p = 0.009; hazard ratio [HR], 1.74), but was independent of the risk associated with post-PV MF or blast-phase disease (HR, 4.83 and 16.63, respectively)
Conclusion
This retrospective analysis showed that vascular events were associated with an acceleratory effect in the development of post-PV MF and blast-phase disease.
The impact of thrombosis on transition probability to death or post-PV MF reduced after 10 years and was observed to reverse MF evolution impact direction at the 12-year timepoint. These observations suggest that thrombosis is a marker of aggressive disease biology or a disease-associated inflammatory state in PV and has important implications for disease progression.
The authors noted several limitations of the study, including selection bias and incomplete data. In particular, data on the impact of cytoreductive therapy on postdiagnosis events were unavailable due to missing information. However, overall, this analysis offers new insights into the relationship between thrombosis and mortality and will inform future clinical practice and investigations regarding MF, blast-phase disease, and mortality risk predictions.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


