All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Impact of TP53 mutations in patients with MF undergoing HSCT
The TP53 gene plays a critical role in cell cycle regulation, apoptosis, and genomic stability. It is considered to be the most frequently mutated gene within the clinical landscape of hematology and oncology; however, the rate of TP53 mutations is highly variable between the types and stages of cancer.1 The role of TP53 in myelofibrosis (MF) prognosis remains poorly understood and has not yet been reported in the setting of hematopoietic stem cell transplantation (HSCT).1 In response to this knowledge gap, Gagelmann et al.1 recently published a study in Blood investigating the impact of TP53 on outcomes in patients with MF undergoing HSCT. We summarize their findings in the article below.
Study design
- 349 patients diagnosed with primary or post-essential thrombocythemia/polycythemia vera MF were enrolled.
- Patients identified as having MF in progression to acute myeloid leukemia were excluded.
- Bone marrow and peripheral blood samples were obtained prior to transplantation, and mutations were analyzed using next-generation sequencing.
- The primary endpoint was overall survival (OS).
- Secondary endpoints included non-relapse mortality, progression-free survival, and leukemic transformation.
Results
The characteristics of all patients are shown in Table 1; 14% of whom had a TP53 mutation.
At baseline, patients with a TP53 mutation
- had a higher incidence of complex karyotypes and unfavorable cytogenetic risk;
- were less likely to receive pre-transplant ruxolitinib therapy; and
- were more frequently administered high-intensity conditioning regimens.
Table 1. Patient characteristics*
|
HSCT, hematopoietic stem cell transplant. |
||||
|
Characteristic, % (unless otherwise stated) |
TP53wt |
TP53mut single-hit |
TP53mut multi-hit |
p value† |
|---|---|---|---|---|
|
Median age (range), years |
58 (22–75) |
52 (44–74) |
58 (24–75) |
0.62 |
|
Female |
40 |
47 |
48 |
0.58 |
|
Diagnosis |
0.23 |
|||
|
Primary myelofibrosis |
71 |
74 |
57 |
|
|
Secondary myelofibrosis |
29 |
26 |
43 |
|
|
Driver mutation |
0.16 |
|||
|
CALR |
20 |
42 |
17 |
|
|
JAK2 |
58 |
37 |
57 |
|
|
MPL |
5 |
5 |
0 |
|
|
Triple negative |
16 |
16 |
27 |
|
|
High molecular risk‡ |
44 |
35 |
35 |
0.54 |
|
Cytogenetic risk |
0.004 |
|||
|
Favorable |
73 |
68 |
50 |
|
|
Unfavorable |
20 |
21 |
50 |
|
|
Very high risk |
7 |
11 |
0 |
|
|
Complex karyotype |
7 |
0 |
47 |
<0.001 |
|
Ruxolitinib pre-HSCT |
42 |
32 |
20 |
0.05 |
|
Conditioning intensity |
<0.001 |
|||
|
Reduced |
87 |
47 |
50 |
|
|
Myeloablative |
13 |
53 |
50 |
|
|
Median time to HSCT (range), months |
26 (0.5–567) |
33 (4.6–266.3) |
14.2 (2.0–356.6) |
0.58 |
- The median variant allele frequency was 20.3%, while 8.6% of patients showed a multi-hit constellation.
- Patients with a TP53 mutation had a similar occurrence of driver mutations compared with patients with wild-type TP53.
- JAK2 mutation was the most frequent, followed by CALR and triple negative.
- There was no statistically significant difference between patients with mutant and wild-type TP53 for high-risk mutations.
- Patients with a multi-hit constellation presented with more severe anemia and thrombocytopenia.
- Across the total cohort, the majority of patients presented with favorable risk, followed by unfavorable risk and then very high risk (Figure 1).
- A complex karyotype was present in a total of 10% of patients.
Figure 1. Total patient cohort risk classification*
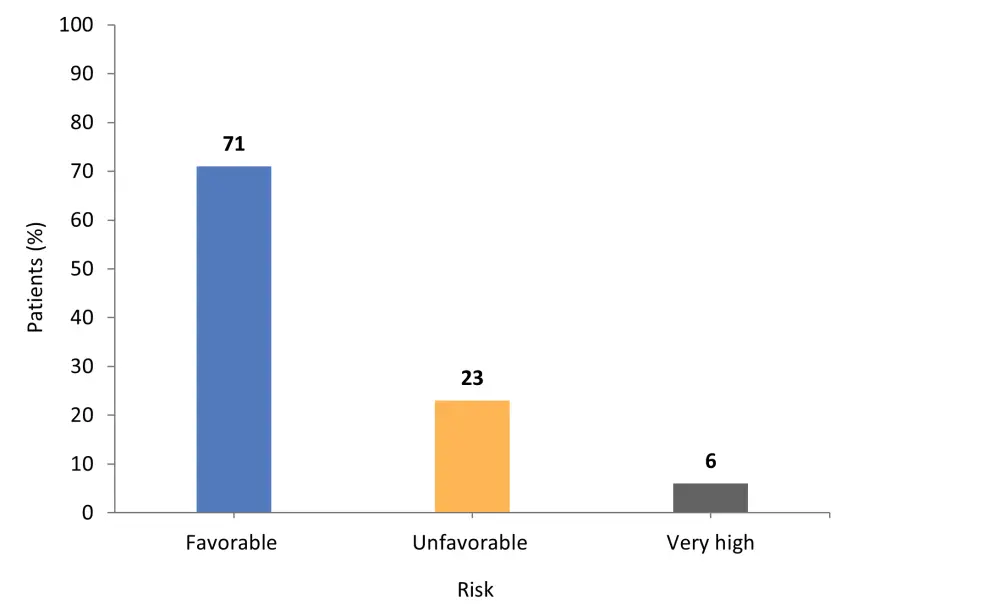
*Adapted from Gagelmann et al.1
- Patients with a TP53 mutation were enriched in the unfavorable risk category at 39%, compared with 20% of patients with wild-type TP53 (p = 0.01).
- For complex karyotype, 29% of patients had the TP53 mutation and 7% had wild-type TP53 (p < 0.001).
Impact of TP53 mutation on HSCT outcomes
The median follow-up for patients with a TP53 mutation was 5.8 years, compared with 9.2 years for patients with wild-type TP53.
The median OS and 6-year OS were significantly lower in patients with a TP53 mutation compared with those with wild-type TP53:
- Median OS, 1.5 years vs 13.5 years
- 6-years OS, 36% vs 64% (p < 0.001)
The 6-year cumulative incidence of relapse (CIR) was significantly higher in patients with a TP53 mutation compared with wild type:
- 39% vs 21% (p = 0.001)
The CIR was also higher in patients with a multi-hit constellation compared with those with a single-hit constellation:
- 52% vs 17% (p = 0.03)
A total of 39% of patients with a TP53 mutation experienced disease relapse, compared with 21% of patients with wild-type TP53. Non-relapse mortality was not significantly different between any of the groups (p = 0.54).
Impact of cytogenetics and TP53
Across the total cohort, cytogenetic risk stratification showed no discrete survival differences (p = 0.40). Patients with favorable cytogenetic risk had the highest 6-year OS rate (Figure 2).
Figure 2. 6-year OS rate according to cytogenetic risk*
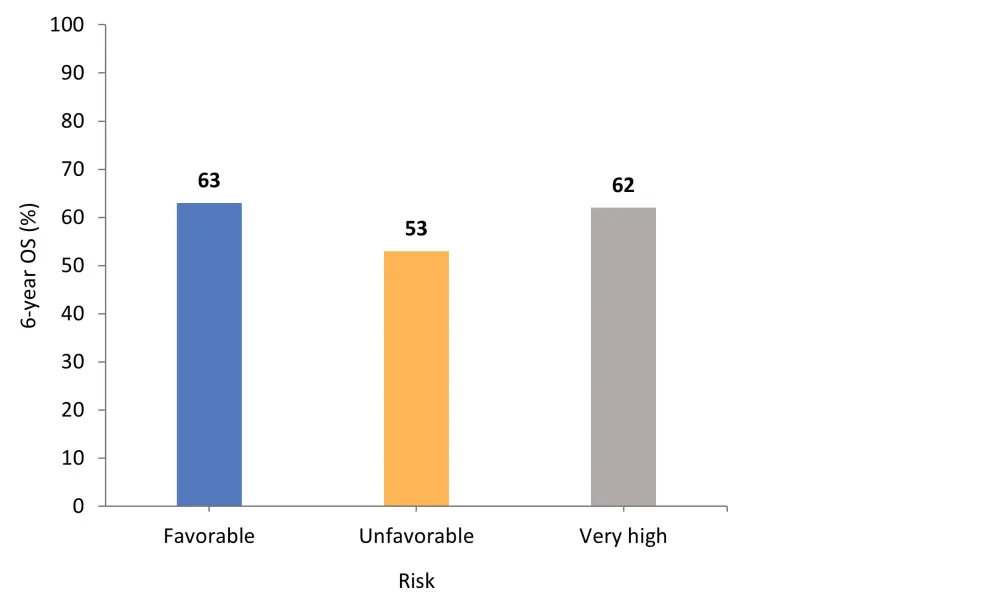
OS, overall survival.
*Adapted from Gagelmann, et al.1
There was significant correlation between complex karyotype and TP53 mutation, with outcomes being heavily impacted by mutation status, especially multi-hit constellation (p < 0.001). By contrast, cytogenetic risk stratification failed to separate patients with different relapse risk (p = 0.73). The 6-year CIR rate for patients with complex karyotypes was 31%, compared with 19% for those with other aberrations (p = 0.03).
Other factors and multivariate analysis
Driver mutation genotype and ASXL1 mutations were the only other variables identified as impacting post-HSCT OS.
Neither dynamic International Prognostic Scoring System (DIPSS) nor mutation-enhanced IPSS (MIPSS70) risk stratification models showed prognostic utility for predicting relapse. However, the Myelofibrosis Transplant Scoring System (MTSS) model was able to separate discrete groups. Multivariate analysis revealed a significant impact of multi-hit constellation on OS after HSCT, whilst single-hit constellation showed similar outcomes compared with patients with wild-type TP53.
No significant difference in OS was reported between different conditioning intensities, and no correlation between TP53 status and platelet count was observed.
Conclusion
The study demonstrated that patients with a TP53 mutation had inferior survival outcomes compared with patients with wild-type TP53. This was driven by multi-hit constellation and resulted in a higher frequency of leukemic transformation and a significantly increased risk of relapse. Patients with mutant TP53 were also more likely to present with severe anemia and thrombocytopenia compared with those with wild-type TP53 or single hit constellation. While selection bias due to the retrospective nature of the study did present as a limitation, the findings remain clinically relevant for both transplant and non-transplant patients. In the future, understanding the mechanisms of TP53 mutations in patients with MF is of high importance in improving outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

