All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
IRENE-G: A prospective RCT of nutritional support following allo-HSCT
Nutrition has a fundamental role in supporting patients with hematologic malignancies at all stages of treatment. Following hematopoietic stem cell transplantation (HSCT), patients with graft-versus-host disease (GvHD) often experience problems with malnutrition, diet, and weight.1,2
The MPN Hub is pleased to summarize the design and rationale of the IRENE-G trial (NCT05111834; Impact of Resistance Exercise and Nutritional Endorsement on physical performance in patients with GvHD), as published in BMC Cancer by Bujan Rivera, et al.2 This is the first prospective trial evaluating the significance of nutrition and exercise for the management of GvHD and clinical outcomes.
The IRENE-G clinical trial
IRENE-G is a 24-week, interventional, randomized, controlled trial exploring the role of nutritional support and exercise in patients with GvHD who have undergone HSCT. The full study design can be seen in Figure 1. The study is expected to run for ~2 years and is aiming to recruit 56 participants to each of two arms (112 total):
- Control arm: nutritional support only during GvHD treatment
- Experimental arm: nutritional support with supervised intervention featuring resistance training (a gradually progressive moderate-to-high intensity exercise)
Figure 1. Design of the IRENE-G clinical trial*
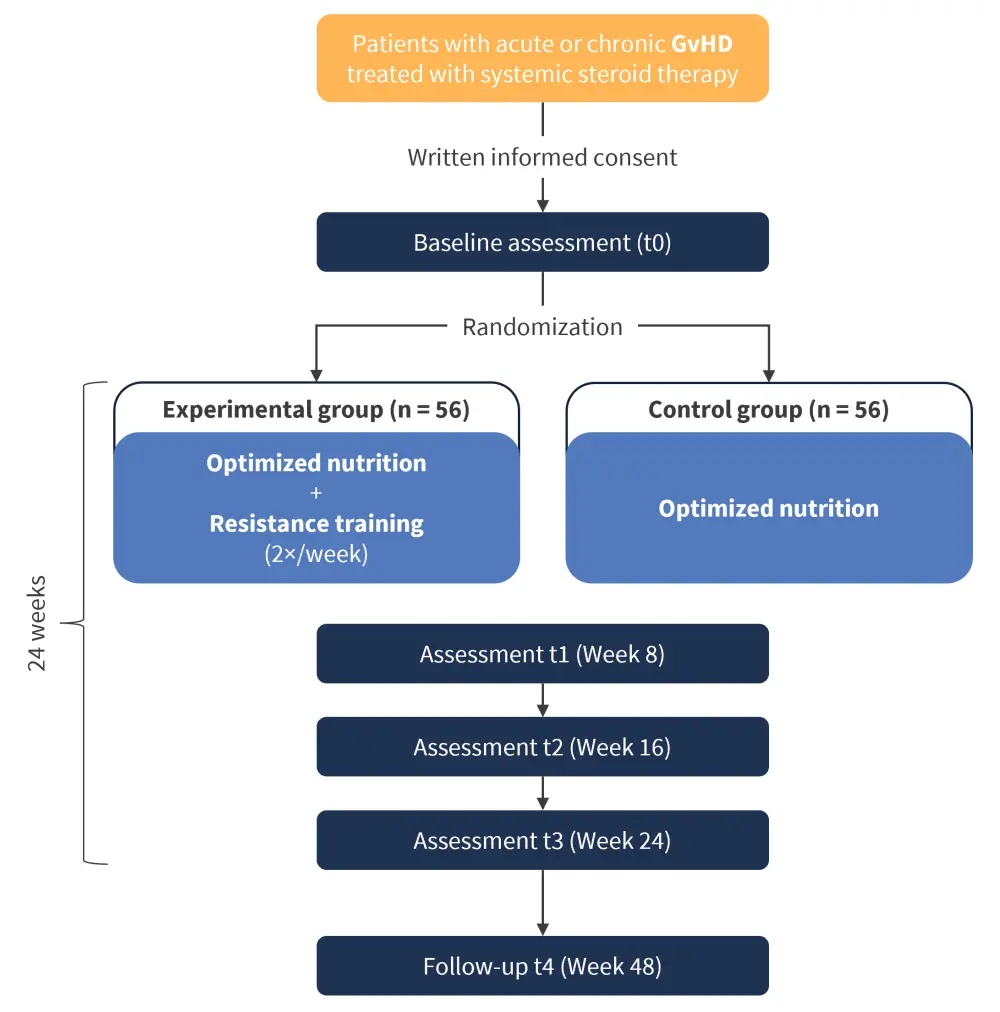
*Adapted from Bujan Rivera, et al.2
The authors hypothesize that, while the physical performance of both study arms should be improved after 24 weeks, the effect will be significantly greater in the experimental arm. It is anticipated that patients in the experimental arm will experience less fatigue, reduced GvHD symptom burden, improved quality of life, increased muscle strength, a better nutritional status as well as better endurance capacity.
The study has clearly defined primary and secondary outcome measures, which will be assessed at the timepoints presented in Figure 2.
Figure 2. Primary and secondary outcomes, assessment methods and measures, and the time points at which they will be assessed*
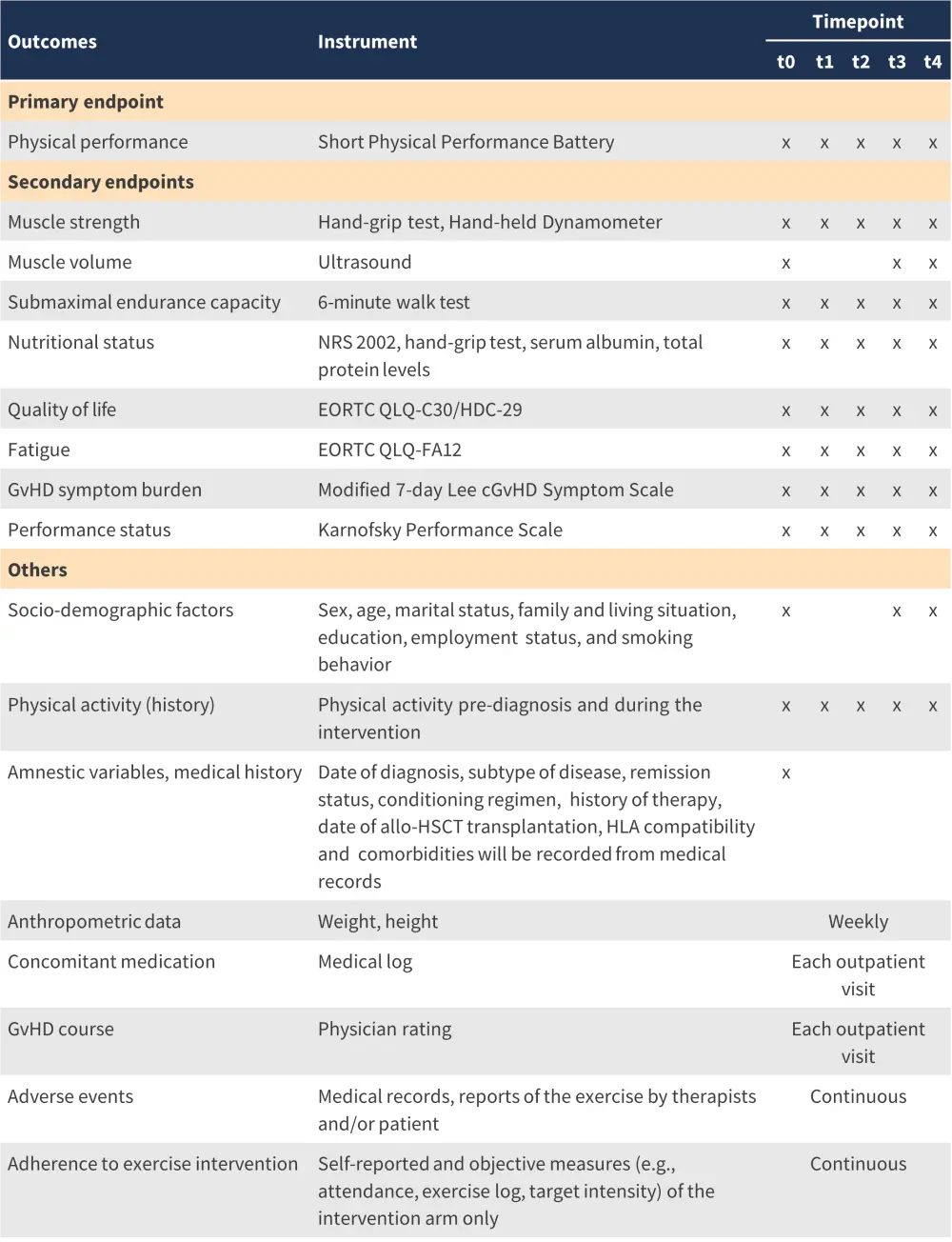
allo-HSCT, allogeneic hematopoietic stem cell transplantation; cGvHD, chronic graft-versus-host disease; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Core Questionnaire; GvHD graft-versus-host disease; HLA, human lymphocyte antigen; NRS, nutrition risk screening.
*Adapted from Bujan Rivera, et al.2
The study also features clear clinical guidelines in the form of a protocol to respond to inadequate calorific intake, as shown in Figure 3.
Figure 3. Protocol for the management of inadequate dietary intake*
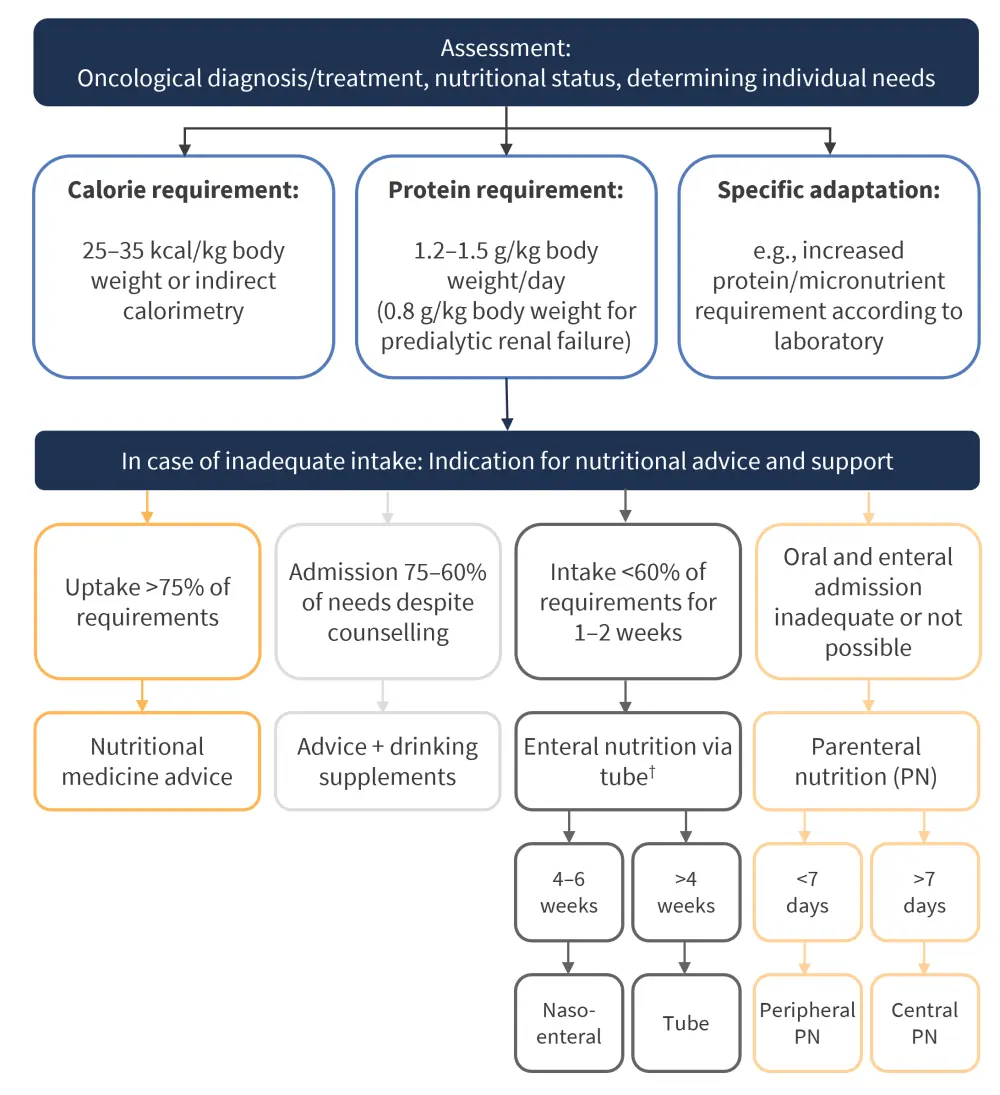
*Adapted from Bujan Rivera, et al.2
†In the absence of patient adherence or contraindication to tube feeding, immediate parenteral nutrition is indicated.
Conclusion
The IRENE-G trial will provide new information on how exercise and nutritional support can benefit patients with GvHD after HSCT. The study is novel in using a clear protocol to respond to inadequate dietary intake, eliminating variability in clinical decision-making about use of interventions. Finally, it will generate evidence of how nutrition can support exercise and physical performance.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

