All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Maternal and fetal risks in pregnancy with MPN
Diagnoses of myeloproliferative neoplasms (MPN) in pregnancy are very rare, occurring in ~3.2 cases per 100,000 pregnancies per year; equivalent to ~24 patients diagnosed with MPN while pregnant over the course of one year.1 Such diagnoses present unique clinical challenges for both the mother and fetus.
Complications associated with the mother include venous and arterial thromboembolism and hemorrhage, while common fetal complications include blood loss, pre-eclampsia, eclampsia, and intrauterine growth retardation.1 Due to the presence of the fetus, standard MPN therapies such as hydroxyurea and ruxolitinib are not suitable for the treatment of pregnant mothers.1 As a result, clinicians currently rely on aspirin, low molecular weight heparin, and pegylated interferon to control any potential complications and maximize the chances of a healthy birth.1
During a 2023 Global MPN Scientific Foundation (GMPNSF) Educational Symposium, Ellis presented findings from a recent systematic review and meta-analysis investigating whether treatment with aspirin, heparin, interferon, or a combination of treatments is associated with live birth rates and reduced adverse maternal outcomes. Below, we summarize the key findings.
Results
- A total of 1,210 pregnant patients with MPN were enrolled in the review.
- The rate of pregnancy loss was 28.7% overall, including
- 28.9% loss in patients with essential thrombocythemia; and
- 33.3% loss in patients with polycythemia vera.
- The highest percentage of patients experienced a pregnancy loss in the first trimester (Figure 1).
Figure 1. Rates of pregnancy loss in each trimester*
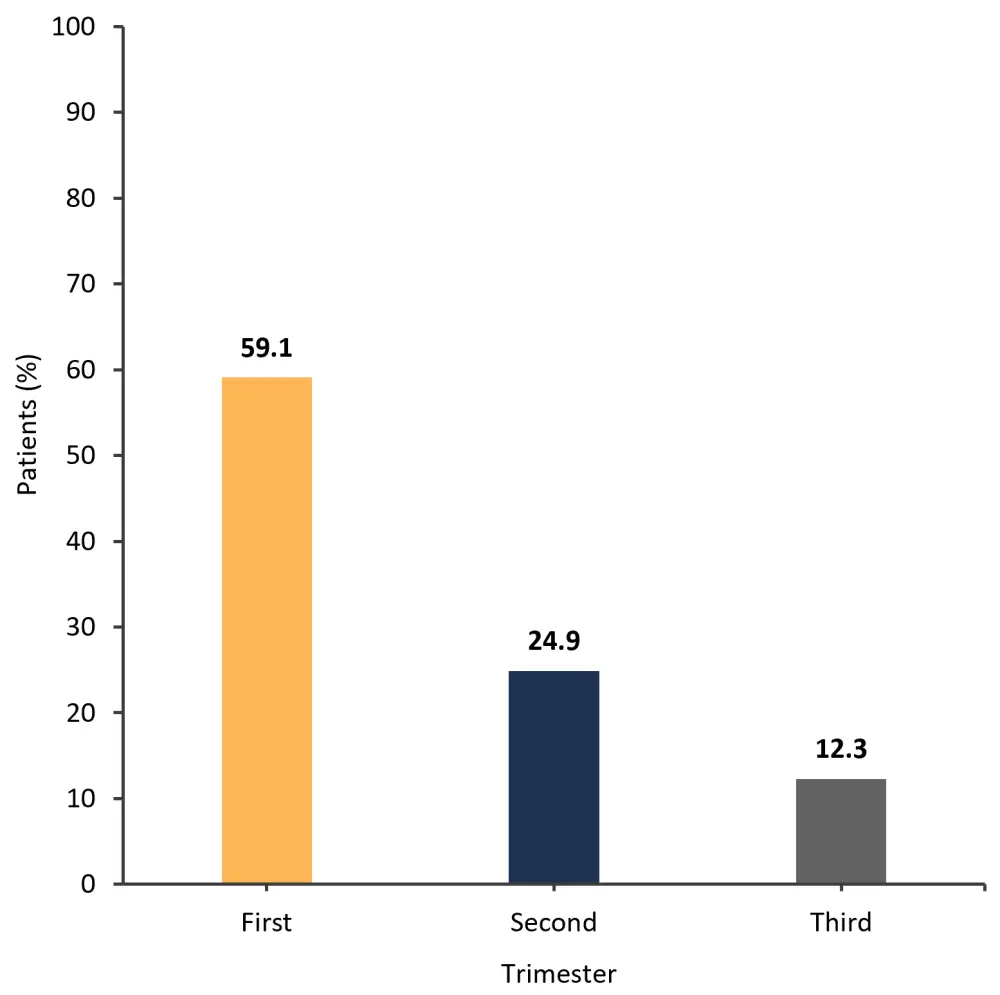
*Adapted from Ellis.1
- Aspirin alone was statistically favored for increasing live birth rate compared with observation alone (hazard ratio [HR], 8.55; confidence interval [CI], 4.03–18.12)
- IFN alone was statistically favored for increasing live birth rate compared with observation alone (HR, 9.72); however, the CI was questionable (2.31–41.01)
- Aspirin plus heparin was favored for increasing live birth rate compared with aspirin alone (HR, 2.13); however, the CI was questionable (0.50–9.03)
- For placenta related complications, aspirin was favored for reducing these events compared with observation alone (HR, 0.59; CI, 0.19–1.84)
- In contrast, aspirin plus heparin compared with observation alone showed no statistical advantage in the use of either treatment approach to reduce the rate of associated complications (HR, 1.07; CI, 0.27–4.28)
Conclusion
Overall, the analysis showed that the live birth rate increased 8–10-fold with aspirin or IFN treatment compared with observation alone. Placenta complications were lower than previously reported. Maternal thrombosis remained uncommon and was unaffected by aspirin or IFN treatment. These findings indicate that the management of pregnancy in MPN patients should remain multidisciplinary, with increased emphasis on the use of uterine artery flow patterns to detect potential abnormalities.
Further investigation into the mechanisms of placenta-mediated complications, different treatment options, and consideration of women with previous pregnancy loss or placenta complications will be important to improving outcomes for these patients.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

