All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Prognostic impact of a cytopenic phenotype in myelofibrosis
Patients diagnosed with primary myelofibrosis (PMF) commonly experience cytopenias, such as anemia, thrombocytopenia, and leukopenia.1
These features are associated with two distinct clinical phenotypes, myeloproliferative (characterized by increased leukocyte and platelet levels) and myelodysplastic (characterized by cytopenias involving one or more hematopoietic lineages). A myelodysplastic cytopenic phenotype (CP) is often associated with poor prognosis.1
Coltro et al.1 recently published a study in Blood Cancer Journal investigating the phenotypic and prognostic correlates associated with a CP in a large cohort of patients diagnosed with PMF.1 The authors placed an emphasis on distinguishing between prefibrotic and overt PMF. We summarize the results below.
Results
Characteristics
The study included 431 patients, of which 216 were diagnosed with prefibrotic PMF and 215 with overt PMF. Patient characteristics are shown in Table 1.
Table 1. Patient characteristics*
|
BM, bone marrow; LCM, left cost margin; LDH, lactate dehydrogenase; PB, peripheral blood; PMF, primary myelofibrosis. |
||||
|
Characteristic, % (unless otherwise stated) |
All patients |
Prefibrotic PMF |
Overt PMF |
p value† |
|---|---|---|---|---|
|
Median age at diagnosis (range), years |
64 (18–90) |
62 (18–90) |
65 (21–89) |
0.0267 |
|
Leukocytes <4 × 109/L |
10 |
6 |
13 |
0.005 |
|
Leukocytes >25 × 109/L |
11 |
9 |
12 |
0.34 |
|
Sex-adjusted moderate anemia‡ |
18 |
10 |
26 |
<0.0001 |
|
Sex-adjusted severe anemia‡ |
13 |
9 |
17 |
0.0094 |
|
Platelets <100 × 109/L |
11 |
8 |
14 |
0.06 |
|
Platelets <50 × 109/L |
4 |
3 |
5 |
0.33 |
|
Platelets >450 × 109/L |
43 |
57 |
28 |
<0.0001 |
|
Peripheral CD34+ |
0.9 |
0.4 |
1.4 |
<0.0001 |
|
PB blasts |
2.1 |
1.7 |
2.4 |
<0.0001 |
|
LDH, U/L |
459 |
335 |
644 |
<0.0001 |
|
Splenomegaly (>5 cm below the LCM) |
62 |
46 |
78 |
<0.0001 |
|
Hepatomegaly |
31 |
24 |
39 |
0.0014 |
|
Constitutional symptoms |
30 |
22 |
38 |
0.0004 |
|
Moderate cytopenic phenotype |
21 |
13 |
29 |
<0.0001 |
|
Severe cytopenic phenotype |
15 |
10 |
20 |
<0.0001 |
|
Cytopenic phenotype with one sole cytopenia |
23 |
16 |
31 |
<0.0001 |
|
Cytopenic phenotype with ≥2 cytopenias |
13 |
7 |
18 |
<0.0001 |
|
JAK2 mutated |
67 |
70 |
64 |
0.23 |
|
CALR mutated |
19 |
17 |
20 |
0.39 |
|
MPL mutated |
6 |
6 |
6 |
0.96 |
|
Triple negative |
10 |
11 |
10 |
0.86 |
|
Double mutated |
2 |
3 |
2 |
0.31 |
|
Abnormal karyotype |
29 |
23 |
36 |
0.0159 |
|
Favorable karyotype |
83 |
87 |
77 |
|
|
Unfavorable karyotype |
11 |
7 |
17 |
0.0475 |
|
Very high-risk karyotype |
6 |
6 |
7 |
|
|
Deaths |
45 |
35 |
55 |
<0.0001 |
|
Leukemic transformation |
12 |
10 |
14 |
0.23 |
Cytopenia types and frequency
The presence of at least one of the following cytopenias was used to define a cytopenic phenotype:
- Leukopenia (leukocytes <4 × 109/L)
- Sex-adjusted anemia (hemoglobin <11 g/dL for males and <10 g/dL for females)
- Thrombocytopenia (platelets <100 × 109/L)
The cytopenia types recorded in both patient groups are shown in Figure 1 and the total number of cytopenias in both patient groups is shown in Figure 2.
Figure 1. Cytopenia types recorded in patients with prefibrotic or overt PMF*
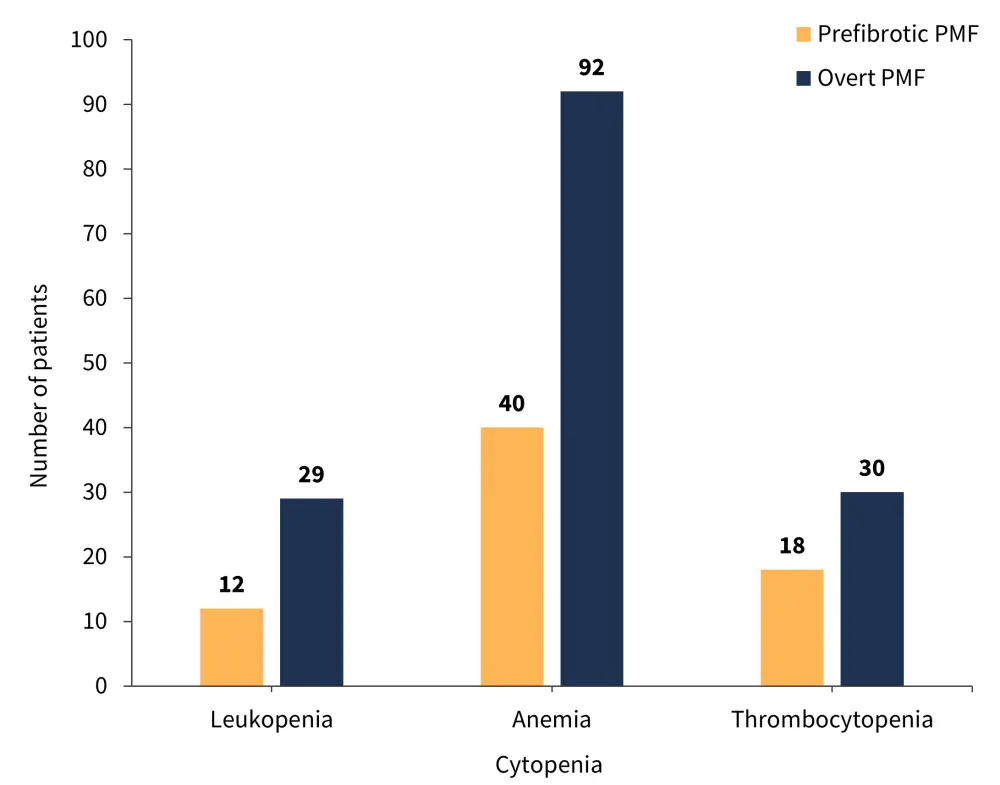
PMF, primary myelofibrosis.
*Adapted from Coltro, et al.1
Figure 2. Number of cytopenias recorded in patients with prefibrotic and overt PMF*
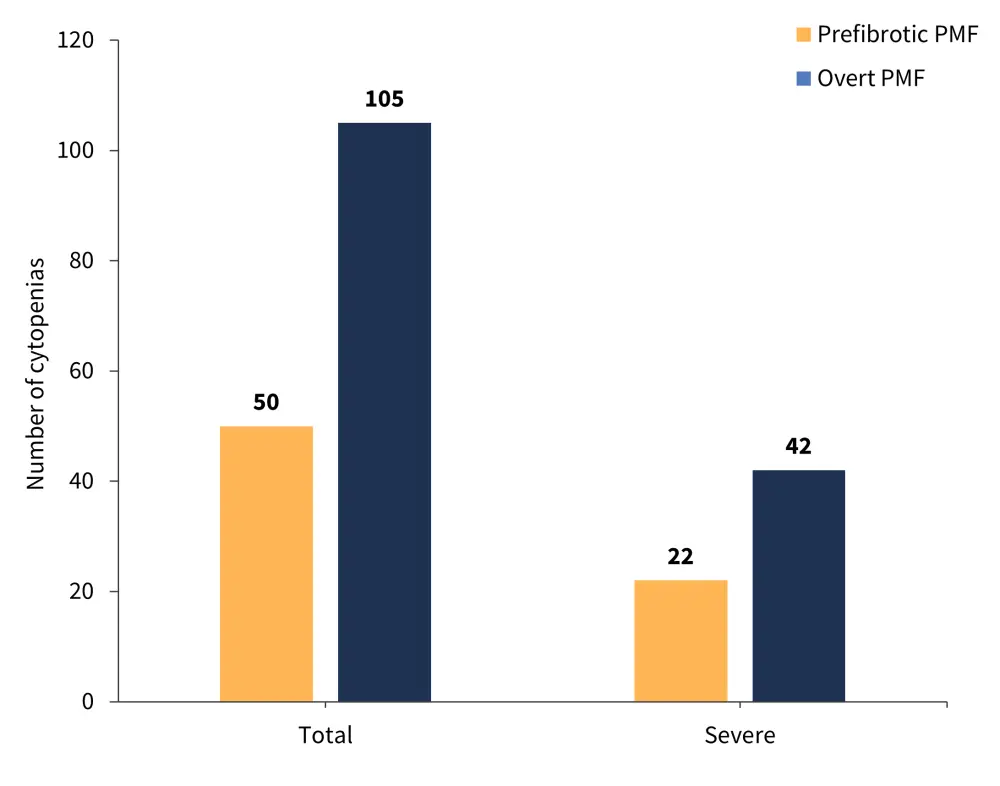
PMF, primary myelofibrosis.
*Adapted from Coltro, et al.1
Associations between a cytopenic phenotype and clinical and genetic factors
Prefibrotic PMF
Patients diagnosed with prefibrotic PMF with a CP were more likely to exhibit the following factors compared with those with a myeloproliferative phenotype:
- Male
- Older age
- Higher peripheral blood blasts and CD34+ cells
- Higher serum lactate dehydrogenase levels
- Higher prevalence of splenomegaly
- Higher prevalence of hepatomegaly
- Constitutional symptoms
- Bone marrow fibrosis (Grade 1)
Patients with a CP were also more likely to have cytogenetic abnormalities and a very high-risk karyotype compared with those with the myeloproliferative phenotype. There was also a higher likelihood of JAK2-unmutated or triple-negative genetic profiles among patients with a CP.
Significantly enriched non-driver mutations in patients with prefibrotic PMF included the following:
- ASXL1
- N/KRAS
- U2AF1
- RUNX1
- SETBP1
- CUX1
- ≥2 high molecular risk mutations
Overt PMF
Patients diagnosed with overt PMF with a CP were associated with the following factors:
- Older age
- Higher CD34+ cell count
- Higher prevalence of bone marrow fibrosis (Grade 3)
- Lower JAK2 mutant burden
- Triple negative
- U2AF1 mutation
Patients with two or more cytopenias had a higher frequency of karyotype abnormalities and mutations in CBL and U2AF1.
Associations between phenotype and survival
Among patients with prefibrotic PMF, a total of 76 (35%) deaths were reported after a median follow-up time of 76 months, with a median overall survival (OS) of 149 months. Patients with prefibrotic PMF and a CP had a significantly worse OS compared with patients with prefibrotic PMF and a proliferative phenotype (p < 0.0001; Figure 3). Patients with a CP were also associated with significantly shorter progression-free survival, at a median of 33 months, compared with 193 months in patients with a myeloproliferative phenotype (p < 0.0001).
For overt PMF, the OS of patients with a CP was significantly shorter compared with those with a myeloproliferative phenotype (p = 0.0026; Figure 3).
Figure 3. Comparison of OS by phenotype and patient group*
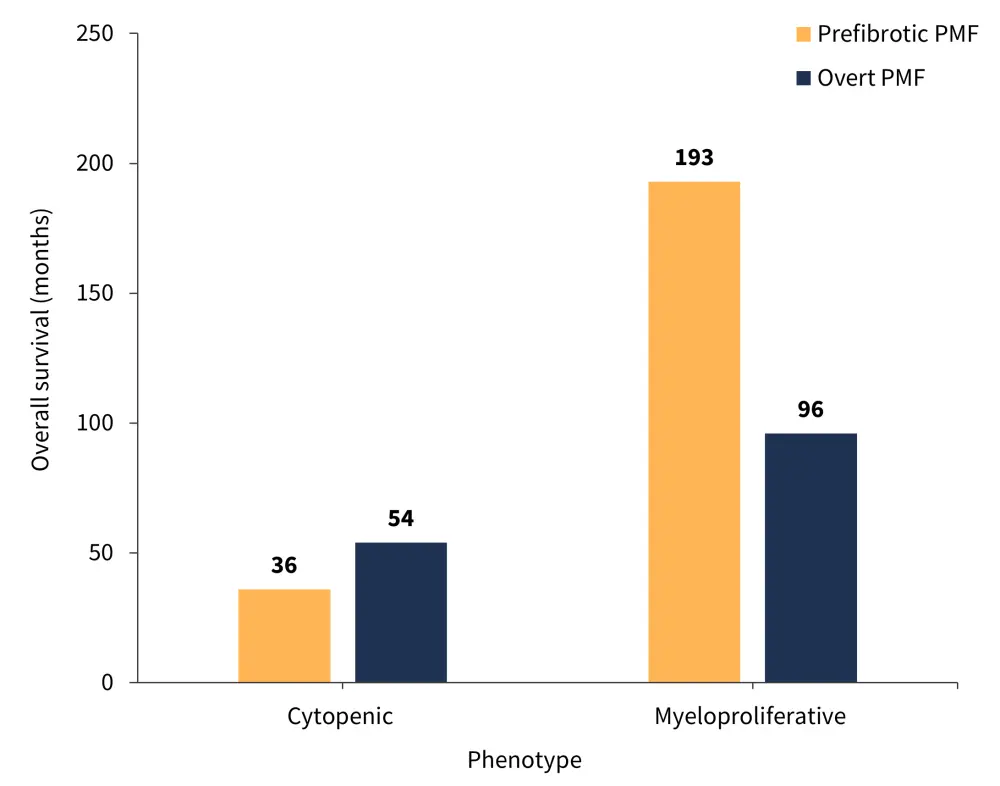
PMF, primary myelofibrosis.
*Adapted from Coltro, et al.1
Moreover, patients with overt PMF and two or more cytopenias experienced a significantly worse OS compared with patients with just one cytopenia (p = 0.0146) and patients with overt PMF and a severe cytopenia were associated with a worse OS compared with those with a moderate cytopenia (p < 0.0001; Figure 4).
Figure 4. Overall survival of patients with overt PMF with A ≥2 cytopenias compared with 1 cytopenia and B a severe cytopenia compared with a moderate cytopenia*
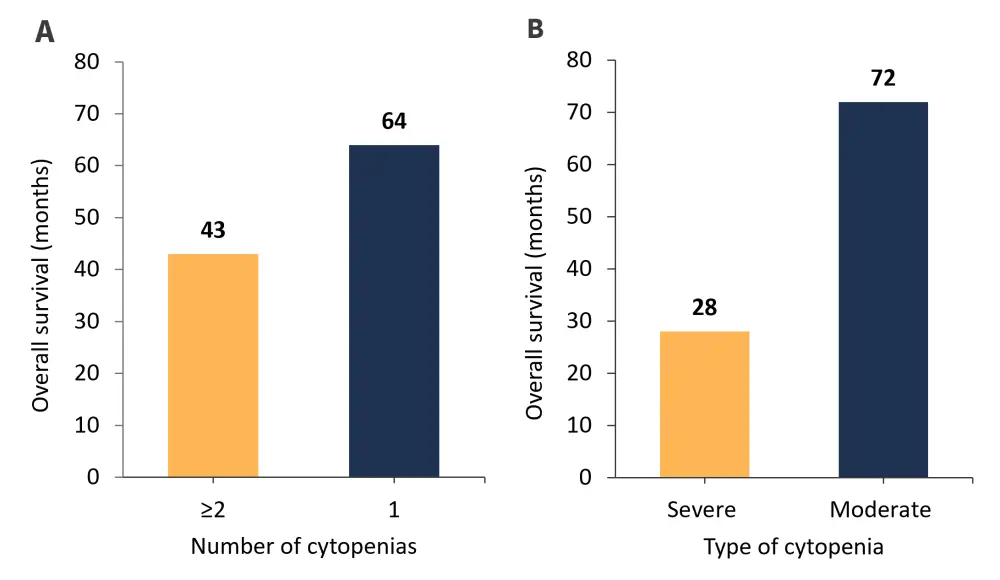
*Adapted from Coltro, et al.1
Multivariate analysis
Among patients with profibrotic PMF, multivariate analysis identified the following factors as independent predictors of a worse OS:
- Moderate/severe anemia
- Leukocytosis
- Constitutional symptoms
- High molecular risk
Among patients with overt PMF, multivariate analysis identified the following independent predictors for inferior OS:
- Severe thrombocytopenia
- Severe anemia
- Peripheral blast count ≥2%
- High molecular risk
- ≥2 high molecular risk associated genes
Leukemic transformation
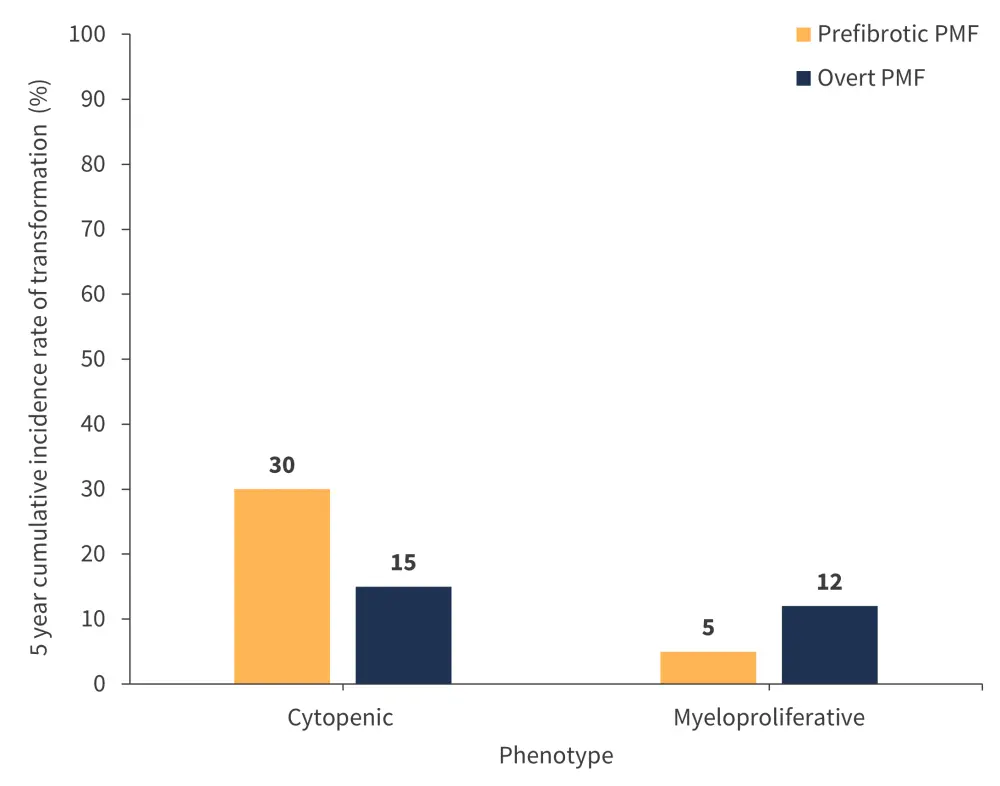
In the study’s most recent follow-up, 20 patients with profibrotic PMF experienced a transformation to acute leukemia. The 5‑year cumulative incidence rate of leukemic transformation in patients with prefibrotic PMF was significantly higher in those with CP compared with those with a proliferative phenotype (p < 0.0001; Figure 5).
Among the patients with over PMF, 28 experienced transformation to acute leukemia. However, the difference in the 5-year cumulative incidence rate of transformation was not statistically different between patients with a CP or a myeloproliferative phenotype (p = 0.4; Figure 5).
Figure 5. 5-year cumulative incidence rate of leukemic transformation by patient group and phenotype*
PMF, primary myelofibrosis.
*Adapted from Coltro, et al.1
Progression of prefibrotic PMF to overt PMF
Patients with prefibrotic PMF with a CP recorded a significantly higher 5-year cumulative incidence rate of progression to overt PMF (67%) compared with patients with a proliferative phenotype (15%) (p < 0.0001). Anemia and thrombocytopenia were also significantly more prevalent among patients with prefibrotic PMF who progressed to overt PMF within 5 years of diagnosis (p < 0.0001 for both anemia and thrombocytopenia; Figure 6).
Figure 6. Incidence of anemia and thrombocytopenia among patients who progressed from prefibrotic PMF to overt PMF within 5 years of diagnosis*
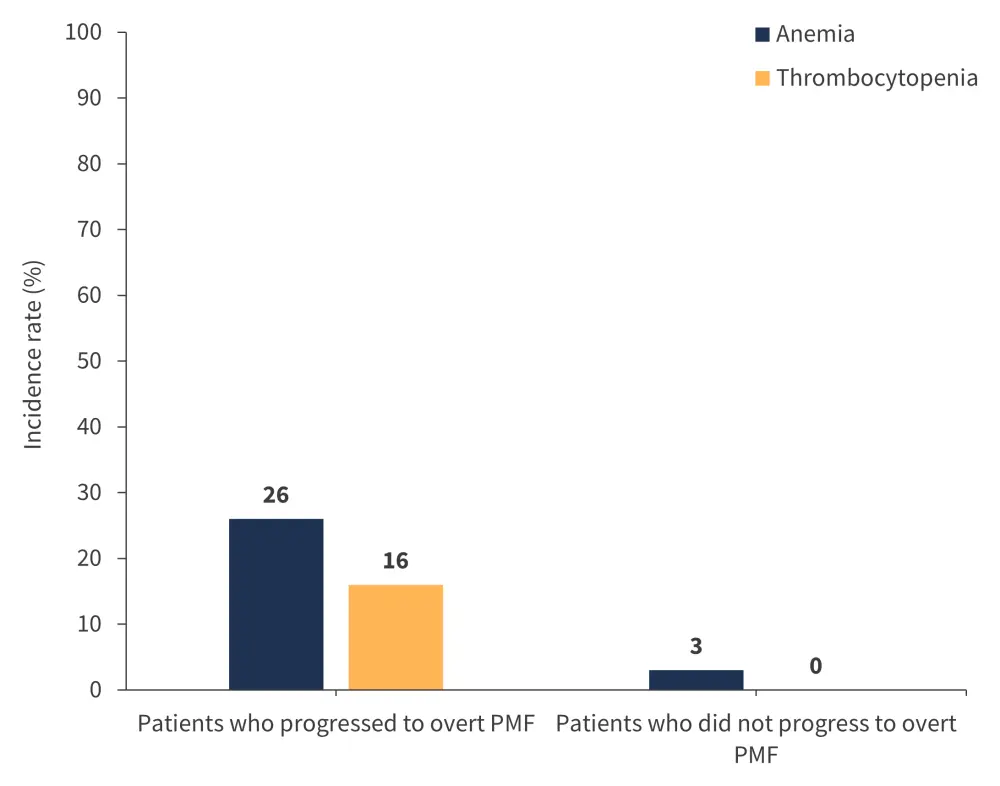
PMF, primary myelofibrosis.
*Adapted from Coltro, et al.1
Conclusion
Results from this study highlight that cytopenic features are more common in patients with overt PMF compared with prefibrotic PMF. Interestingly, mutations in U2AF1 were found to be a prominent abnormality of a CP in both prefibrotic and overt PMF. Importantly, the results also demonstrated that a CP is associated with a significantly reduced OS in both PMF subtypes, a higher risk of leukemic transformation, and fibrotic progression for patients with prefibrotic PMF. The authors highlight that a CP analysis is minimally invasive for patients, simple to achieve, and can be performed over an extended period.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

