All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Editorial theme | Progression in myeloproliferative neoplasms
Do you know... Which of the following is a common clinical manifestation of MPN progression?
This editorial theme article will discuss the clinical characteristics of progression of myeloproliferative neoplasms (MPN) and the risk factors associated with progression. To review this subject, two articles will be summarized. The first, by Sabattini, et al.1, looked at pathological features of progression, and the second, by Baumeister, et al.2, looked at MPN progression through the lens of diagnostic and prognostic features.
MPN progression
Clinical manifestations
Clinically apparent signs of MPN progression include1:
- Development or worsening of myelofibrosis (MF)
- Changes in blood counts (e.g., increased white blood cells, decreased red blood cells, or the appearance of granulocytic blasts or precursors)
In terms of symptoms, a patient might experience the onset or increase of thromboembolic events, bleeds, or other symptoms, including itching, night sweats, pyrexia, cachexia, and fatigue.2
Fibrotic progression
Fibrosis is a dynamic process that results in remodeling of the bone trabeculae and develops progressively.1 In MPN, MF development or progression is one of the most frequent features and Table 1 shows the essential and additional diagnostic criteria used.2 The 0−3 grading system listed in this table is the most widely used and comes from the European Consensus on grading bone marrow fibrosis. Grades 0−3 are defined as follows3:
- Grade 0: Scattered linear reticulin with no intersections corresponding to normal bone marrow
- Grade 1: Loose network of reticulin with many intersections, especially in perivascular areas
- Grade 2: Diffuse and dense increase in reticulin with extensive intersections, occasionally with only focal bundles of collagen and/or focal osteosclerosis
- Grade 3: Diffuse and dense increase in reticulin with extensive intersections with coarse bundles of collagen, often associated with significant osteosclerosis3
Table 1. Diagnostic criteria for post-ET and post-PV MF from the WHO*
|
ET essential thrombocythemia; MF, myelofibrosis; PV, polycythemia vera; WHO, World Health Organization. |
|
|
Diagnostic criteria for post-ET MF |
|
|---|---|
|
Required criteria |
Documentation of previous diagnosis of WHO-defined ET |
|
Bone marrow fibrosis of Grade 2–3 on a 0–3 scale |
|
|
Additional criteria (two needed) |
Anemia (i.e., below the reference range given age, sex, and altitude considerations) and a >2 g/dL decrease from baseline hemoglobin concentration |
|
Leukoerythroblastosis |
|
|
Increasing splenomegaly, defined as either an increase in palpable splenomegaly of >5 cm from baseline (distance from the left costal margin or on imaging) or the development of a newly palpable splenomegaly |
|
|
Raised lactate dehydrogenase level above the reference range |
|
|
Development of any two (or all three) of the following constitutional symptoms: >10% weight loss in 6 months, night sweats, and unexplained fever (>37.5°C) |
|
|
Diagnostic criteria for post-PV MF |
|
|
Required criteria |
Documentation of previous diagnosis of WHO-defined PV |
|
Bone marrow fibrosis of Grade 2–3 on a 0–3 scale or 3–4 on a 0–4 scale |
|
|
Additional criteria (two needed) |
Anemia (i.e., below the reference range given age, sex, and altitude considerations) or sustained loss of requirement of either phlebotomy (in the absence of cytoreductive therapy) or cytoreductive treatment for erythrocytosis |
|
Leukoerythroblastosis |
|
|
Increasing splenomegaly, defined as either an increase in palpable splenomegaly of >5 cm from baseline (distance from the left costal margin) or the development of a newly palpable splenomegaly |
|
|
Development of any two (or all three) of the following constitutional symptoms: >10% weight loss in 6 months, night sweats, and unexplained fever (>37.5°C) |
|
Differentiating between post-essential thrombocythemia (ET), post-polycythemia vera (PV) MF, and overt primary myelofibrosis (PMF) can be difficult as they are morphologically quite similar. To assess fibrotic progression, it is important to consider a patient’s history of chronic phase MPN.1
Leukemic progression
Progression of MPN to acute leukemia is possible, with two forms being recognized. If a patient has between 10% and 19% blasts in the bone marrow or peripheral blood, this is termed MPN‑accelerated phase (AP), whereas >20% blasts is termed MPN-blast phase (BP), also known as acute myeloid leukemia (AML),1,2 as shown in Figure 1. Progression to AML/MPN-BP is associated with a greatly decreased survival (median overall survival of 3.6 months).2 The prognosis for patients that progress to AML is worse than for patients who develop de novo AML.1 Progression from ET to MPN‑AP or MPN‑BP is associated with the lowest risk (0.7−3% at 10 years), whereas for patients with PV the risk is a little higher at 2.3−14.4% at 10 years.1
Figure 1. Summary of MPN progression*†
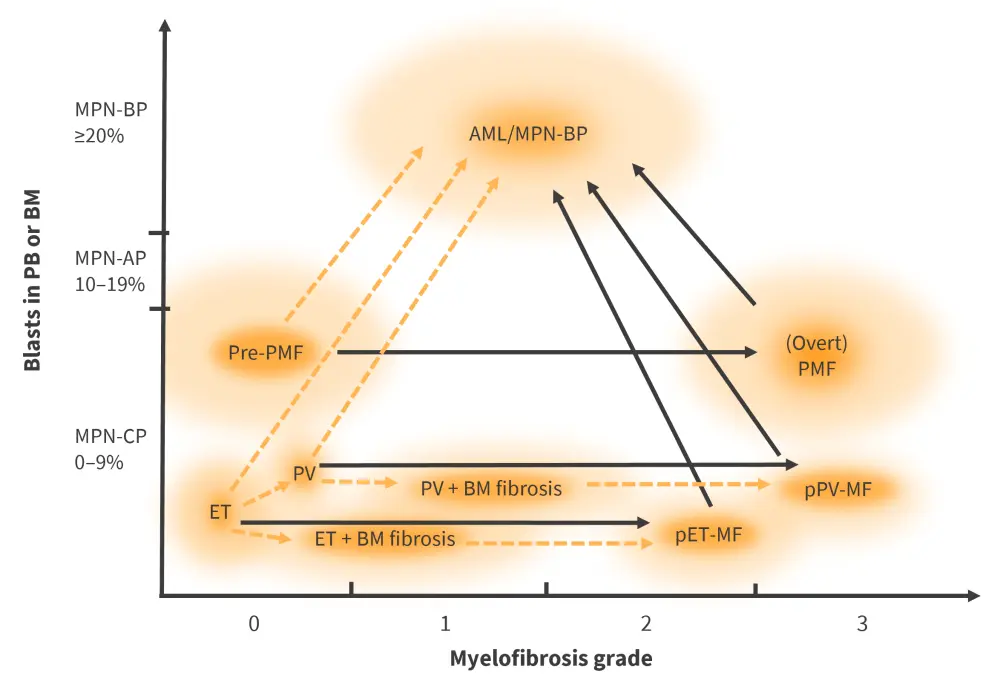
AML, acute myeloid leukemia; BM, bone marrow; ET, essential thrombocythemia; MPN, myeloproliferative neoplasms; MPN-AP, MPN‑accelerated phase; MPN-BP, MPN-blast phase; MPN-CP, MPN-chronic phase; PB, peripheral blood; pET-MF, post-ET myelofibrosis; PMF, primary myelofibrosis; pPV-MF, post-PV myelofibrosis; pre-PMF, prefibrotic primary myelofibrosis; PV, polycythemia vera.
*Adapted from Baumeister, et al.2
†Black arrows indicate more common progression pathways, whereas orange dotted lines indicate less frequent progression pathways.
Atypical progression features1
While certain clinic-pathologic changes may occur that cannot be classified under the current WHO criteria, these conditions may represent disease evolution rather than presentation of mixed features of disease.
Monocytosis or leukocytosis in rare instances may precede and lead to the acquisition of myelodysplastic/MPN-like features. In patients with PMF, monocytosis has been reported in 5–15%. In this instance, a greater degree of/or persistent leukoerythroblastosis may allow for differentiation between PMF and de novo chronic myelomonocytic leukemia (CMML). Patients with PMF or CMML have shared features such as JAK2 V617F mutation, monocytosis, and sometimes fibrosis, which can make differentiation tricky.
Features common to JAK2 V617F-positive PMF with monocytosis that can help to separate patients from others with CMML include:
- Mean increased JAK2 V617F allelic burden (median, 43%; range, 20–62%)
- The presence of atypical megakaryocytes
- A large myeloid left shift at blood count
- Difference in the MO1 monocyte fraction comprising CD14+/CD16− cells
- Patients with MPN <92% (mean, 77%)
- Patients with CMML ≥94% (mean, 96.5%)
In patients with MPN or PMF, the presence of monocytosis is a negative prognostic feature and this negative impact has been described to act dose dependently.
While there have been cases of patients presenting with intermediate features that fall between CMML and PMF and do not quite fit into the WHO criteria, these remain rare.
Leukocytosis at onset >15 x 109/L is a risk factor for thrombosis in patients with PV or ET and in the former it is associated with a worse survival and increased risk of leukemic transformation. Rarely, neutrophilia has been described in patients (especially with PV) with advanced stage disease who did not present with leukocytosis at onset.
Polycythemic transformation is rare in JAK2-mutated ET. Some confusion initially arose as various studies reported an incidence of polycythemic progression ranging between 1.4% and 11%. The identification of masked-PV that occurs in patients who present with ET-like features but then show an increase in hemoglobin values during the disease course and/or PV-like bone marrow features helped clear up the situation in part. True cases of erythrocytosis in JAK2-mutated ET do occur but are very low in incidence.
Predictive factors of MPN progression
Inflammation is thought to play a role both in triggering symptoms in patients with MPN and in progression to MF or MPN-BP.2 Fibrotic remodeling can be driven by interaction between the malignant clone and other cells (both hematopoietic and non-hematopoietic) and inflammatory cytokines in the bone marrow environment. Megakaryocytes are thought to play a key role in this process by secreting multiple inflammatory cytokines that trigger the development of myofibroblasts from endothelial or mesenchymal stromal cells. These myofibroblasts then go on to form fibrotic tissue.2
Leukemic progression of patients with PMF to MPN-BP was associated with high levels of interleukin (IL)-8 and IL-1β, while TNF-α was associated with progression to BP regardless of MPN subtype.2 In patients with PMF, increased IL-2R and IL-8 is correlated with poorer survival times.2
Comorbidities, such as kidney disease, may affect the frequency at which patients present with hemorrhagic and thrombotic complications. However, the impact these comorbidities have on the rate of progression of an MPN is not known.2
MF progression
No association has been found between mutations in TET2 and DNMT3A and the risk of fibrotic progression.2
Risk factors for MF progression include the following1:
- Older age
- Extended disease duration
- Increased disease burden (comprising leukocytosis, thrombocytopenia, anemia, and palpable splenomegaly)
- Elevated JAK2 allele burden in PV
- SRSF2, U2AF1, SF3B1, IDH1/2, EZH2, and ASXL1 mutations
- Cytogenetic abnormalities (12p abnormality, acquired loss of heterozygosity of chromosome 1p)
- Resistance to treatment in ET, particularly hydroxyurea4
Genetic risk factors for leukemic progression1
The underlying mechanisms that result in leukemic progression are currently unclear due to the extended duration between the appearance of key mutations and the evolution of the predominant clone. Generally, the number of mutations present tends to increase with progression and in turn has an effect on prognosis. However, specific mutated clones present in chronic phase MPN may act as drivers for this transformation, for example, Williams, et al.5 found that the JAK2 V617F mutation may be acquired as early as in childhood or even in utero.
There is a high degree of variation in the allelic burden of driver mutations found in patients with MPN. Patients with triple-negative MF (no mutations in JAK2 V617F, CALR, or MPL) show an elevated risk of leukemic transformation. The presence of a JAK2 mutation in patients with PMF who also carry driver gene mutations is associated with a greater risk of progression to MPN-BP. CALR mutations on the other hand may be associated with a positive impact on prognosis, providing it is not present with ASXL1 mutations. The role of ASXL1 itself is still not fully clear, with some researchers claiming its negative prognostic impact may only occur when found in patients also harboring mutations in TP53 and other high-risk genes. Up to 35% of patients at transformation show mutation or loss of TP53, particularly in MPN-BP.
Other risk factors for leukemic progression
Leukemic progression risk factors include the following:
- Advanced age
- Severe anemia
- Leukocytosis
- Circulating blasts >2%
- Thrombocytopenia
- Advanced bone marrow fibrosis
- Cytogenetic abnormalities
- Acquisition of ≥2 high-risk mutations
- Monocytosis (risk factor for MPN-AP progression)
<10% blasts as a prognostic feature6
While the WHO definition of leukemic progression does not include <10% blasts as a factor, there is evidence that patients showing increased blasts that do not exceed this threshold may have poorer prognosis than those with <5%. In a study by Geyer, et al.6, 92 patients diagnosed with MPN, including PMF, PV/post-PV MF, and ET/post-ET MF, were split into three groups according to the percentage of blasts present. These groups were as follows:
- IB-1: 2–4% peripheral blood and <5% bone marrow blasts
- IB-2: 5–9% marrow and/or peripheral blood blasts
- AP: ≥10–19% marrow or peripheral blood blasts
Compared with the IB-1 and control group patients, the IB-2 group showed significantly decreased overall survival (OS; p = 0.0084 and p < 0.0001, respectively). The combined OS of the IB-1 + IB-2 group was longer than that of the patients in the AP group. Other studies confirm this observation that >3% blast count is associated with an increase in the risk of leukemic transformation in the first 5 years since diagnosis in patients with PMF.1
Conclusion
MPN are a dynamic group of diseases that may change their clinical features over time or progress to a different category, which makes their classification and diagnosis a challenge. Understanding how MPN progression occurs, and the risk factors associated with progression will aid in predicting a patient’s prognosis and may allow the development of better strategies to prevent progression. Currently, treatment in most patients will decrease the disease symptoms while not altering the underlying disease course and therefore not impacting survival. Therefore, the development of treatments that can slow or stop progression are also a key focus for future work.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

