All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Real-life diagnostic and treatment approaches for patients with MPN
Implementation of the revised 2016 World Health Organization (WHO) classification for myeloproliferative neoplasms resulted in the introduction of the pre-fibrotic phase of primary myelofibrosis (MF).1 These changes had implications for the treatment paradigm and may impact interpretations of data from clinical trials completed prior to the revised classification.1
A retrospective chart review published by Schimdt et al.1 in the Journal of Cancer Research and Clinical Oncology evaluates real-life approaches to clinical characteristics, diagnostic assessment, risk stratification, and treatment decisions in patients diagnosed with essential thrombocythemia (ET) or MF following the introduction of the revised WHO 2016 classifications. We summarize the key findings from this review here.
Results
The retrospective chart review took place in 31 clinical centers across Germany between April 2021 and May 2022. A total of 960 patients were included in the analysis; 495 patients diagnosed with ET and 465 patients with MF (Table 1).
Table 1. Clinical and molecular characteristics of patients with ET and MF*
|
ET, essential thrombocythemia; Hb, hemoglobin; LDH, lactate dehydrogenase; MF, myelofibrosis; WBC, white blood cell. |
||
|
Characteristic, % |
ET |
MF |
|---|---|---|
|
Sex |
|
|
|
Male |
44.6 |
49.5 |
|
Female |
55.4 |
50.5 |
|
Age |
||
|
<50 years |
13.7 |
7.7 |
|
<50–60 years |
18.6 |
13.3 |
|
>60–70 years |
21.0 |
26.7 |
|
>70 years |
46.1 |
52.3 |
|
Driver mutation |
||
|
JAK2V617F |
74.1 |
76.9 |
|
CALR |
22.2 |
18.7 |
|
MPL |
6.4 |
7.0 |
|
Palpable splenomegaly |
4.4 |
22.4 |
|
LDH |
||
|
Below upper normal |
14.5 |
9.7 |
|
Above upper normal |
80.4 |
78.7 |
|
Blasts in peripheral blood |
||
|
≥1% |
3.6 |
7.7 |
|
<1% |
50.7 |
13.3 |
|
WBC count |
||
|
≥11.000/µl |
27.1 |
42.4 |
|
<11.000/µl |
70.9 |
53.8 |
|
Anemia |
||
|
Severe (Hb <10 g/dl) |
2.0 |
14.0 |
|
Mild (Hb ≥10 g/dl and <12 g/dl) |
12.5 |
22.2 |
|
Platelet count |
||
|
≥450.000/µl |
93.7 |
62.4 |
|
100.000–450.000/µl |
4.4 |
31.6 |
|
<100.000/µl |
0.2 |
1.9 |
More than 80% of patients with ET showed ≥1 diagnostic minor criterion for MF and over 50% of patients with MF exhibited criteria consistent with the pre-fibrotic phase. Interestingly, only 58.6% of patients diagnosed with ET received a bone marrow (BM) biopsy at the time of initial diagnosis, whilst 91.8% of patients with MF received BM histology.
Symptom burden
Patients diagnosed with myeloproliferative neoplasms commonly report negative impacts on social interactions, productivity, physical activity, and quality of life due to their disease-related symptoms (Figure 1). However, the study indicated that symptom burden does not necessarily correlate with disease subtype.
Figure 1. Symptom burden in patients with ET and MF*
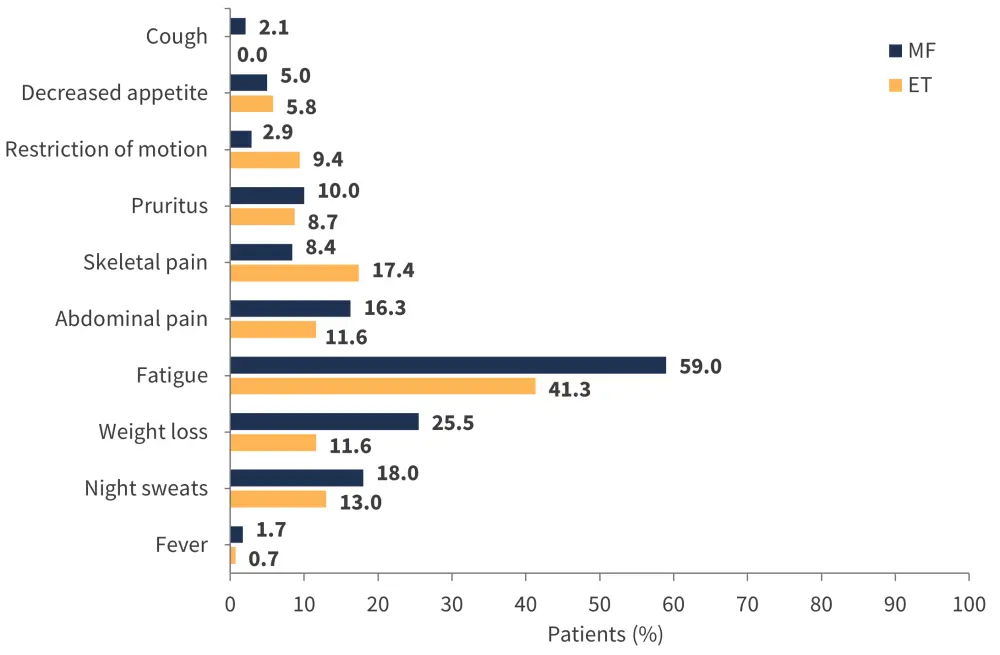
ET, essential thrombocythemia; MF, myelofibrosis.
*Adapted from Schimdt, et al.1
Risk stratification
Of the total patient cohort, 35.3% of patients received an MF risk stratification score. Patients diagnosed with primary MF were stratified using the following scoring systems:
- International Prognostic Scoring System (IPSS; 37.8%)
- Dynamic International Prognostic Scoring System (DIPSS; 24.4%)
- Dynamic International Prognostic Scoring System plus (DIPSS+; 16.5%)
Patients diagnosed with secondary MF were stratified according to the Myelofibrosis Secondary to Polycythemia Vera and ET-Prognostic Model (MYSEC-PM; 11.6%).
Molecular scoring was used in <8% of cases. The distribution of associated risk categories across the patient cohort is shown in Figure 2. Prognosis score assessment was performed in only 12.9% of patients at later time points; however, dynamic and molecular scoring models were more frequently used at the time of primary diagnosis.
Figure 2. Distribution of molecular risk categories for the total patient cohort*
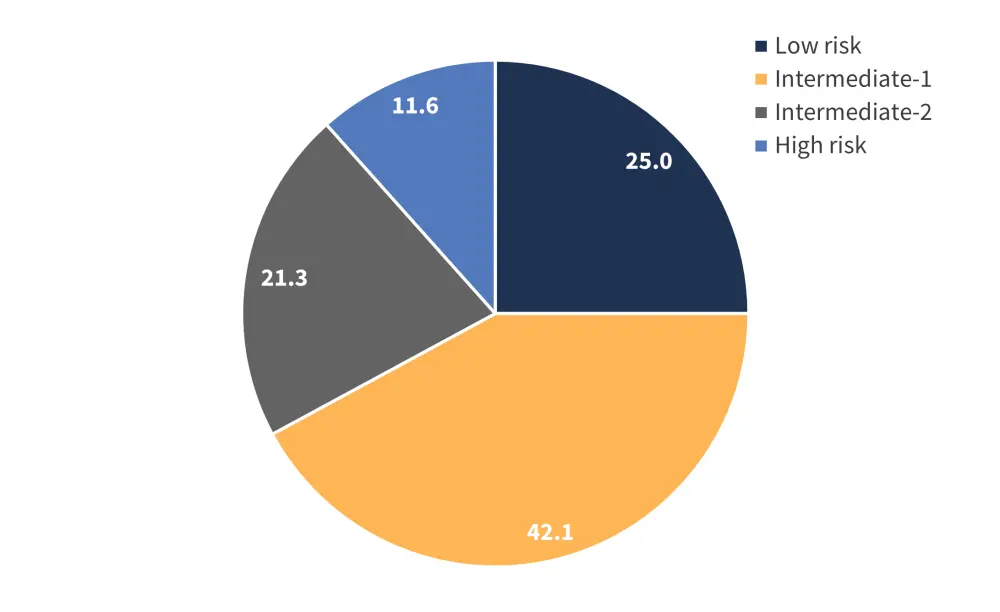
*Adapted from Schimdt, et al.1
Therapeutic strategies
The chart review assessed cardiovascular risk, use of anticoagulants, and disease-specific therapeutic strategies with regards to thromboembolic complications.
- Comorbidities were present in 70.5% of patients with ET and 79.1% of patients with MF.
- Cardiovascular comorbidities were present in 76.5% of patients with ET and 74.2% of patients with MF.
The therapeutic strategies used for patients with ET and MF are shown in Table 2. Pharmacologic therapy was focused on cytoreduction through the use of hydroxycarbamide, while anticoagulant therapy was used less frequently.
Table 2. Therapeutic strategies used in patients with ET and MF*
|
ET, essential thrombocythemia; MF, myelofibrosis. |
||
|
Therapeutic strategies, % |
ET |
MF |
|---|---|---|
|
Anticoagulant therapy |
56.8 |
38.1 |
|
Pharmacologic cytoreduction |
84.7 |
53.1 |
|
Watchful waiting |
21.4 |
22.2 |
|
Transfusion dependent |
2.4 |
8.4 |
Conclusion
Results from the chart review highlighted that 40% of patients diagnosed with ET did not receive histological BM testing at diagnosis and that 60% of patients diagnosed with MF did not receive early prognostic risk assessment. These findings suggest that increased use of histopathology assessment and dynamic risk stratification would improve precise risk analyses and therapeutic stratification. Strategies to implement such changes are yet to be defined.
A potential future strategy in histopathology is the incorporation of artificial intelligence (AI), which provides several potential benefits and applications. AI can be used to both support and speed up clinical reporting, as well as measure morphological features with impartiality.2 It may also offer pathologists the opportunity to focus on more challenging patient cases whilst meeting an ever-increasing workload demand.2 Pathologists and laboratory technicians are expected to remain at the forefront of diagnosis, but with the added support of AI to improve efficiency, resource allocation, cost-effectiveness, and uniform pathology reviews.2
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

