All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Real-world study identifying unmet needs in adolescents and young adults with MPN
Do you know... According to a recent retrospective study, which is the most predominant mutation associated with MPN in children and young adults with MPN?
Treatments for myeloproliferative neoplasms (MPN) are currently adapted to risk classification based on age and history of thrombosis.1 Most data around current therapeutics are gained from patients aged >60; the rarity of MPN in patients aged <25 years means there is a lack of knowledge around disease management, vascular complications, and disease transformation in this age group.1
To address this unmet need, Sobas, et al.1 performed a retrospective study in collaboration with members of the European Hematology Association Scientific Working Group (EHA-SWG) on MPN, with the aim of understanding the risks in contemporary young patients with MPN. We are pleased to provide a summary of their findings here.
Study design
Sequential patients diagnosed with MPN before the age of 25 years were included; suspected or confirmed hereditary cases were excluded. Treatment type and rationale, venesection, antithrombotic, and the use of cytoreductive drugs were assessed. Outcomes including hemorrhage-free survival, overall survival, and thrombosis-free survival were analyzed using established international prognostic scores.
Results
A total of 444 patients from 15 countries were included, with the majority diagnosed between 1990 and 2019 (Table 1). The median follow-up was 9.7 years. A total of 149 patients were asymptomatic. The most common MPN in this age group was essential thrombocythemia (ET).
Table 1. Patient characteristics at diagnosis*
|
ET, essential thrombocythemia; MF, myelofibrosis; MPN, myeloproliferative neoplasms; PV, polycythemia vera. |
||||
|
Characteristic, % (unless otherwise stated) |
Overall |
ET |
PV |
“Other” MPN† |
|---|---|---|---|---|
|
Female |
72.3 |
78 |
48.1 |
75.6 |
|
Median age (range), years |
20.4 |
20.6 |
19.7 |
20.1 |
|
Symptoms |
||||
|
Plethoric face |
3.9 |
0.7 |
21.3 |
0 |
|
Aquagenic pruritus |
5.6 |
3.2 |
19.7 |
0 |
|
Hyperviscosity |
34.5 |
34.2 |
42.2 |
23.7 |
|
Microvascular |
10.8 |
11.6 |
5.8 |
14 |
|
Constitutional |
6.2 |
3.9 |
9.2 |
16.7 |
|
Palpable splenomegaly |
20.3 |
13.6 |
39.1 |
37.2 |
|
Fatigue |
19.8 |
16.5 |
34.5 |
20.9 |
|
Cardiovascular risk |
12.9 |
12.2 |
15.4 |
13.6 |
|
Blood parameters, median (range) |
||||
|
Leucocytes (× 109/L) |
9 |
8.9 |
9.8 |
10.3 |
|
Hemoglobin (g/L) |
140 |
139 |
174.5 |
132 |
|
Platelets (× 109/L) |
863 |
915 |
601 |
775 |
|
Mutations |
||||
|
JAK2V617F |
56 |
48.5 |
86.4 |
55.6 |
|
JAK2 ex12 |
1.13 |
0 |
6.2 |
0 |
|
JAK2 allele burden, |
22 |
15.5 |
29 |
31.8 |
|
CALR |
13.3 |
14.5 |
0 |
28.9 |
|
MPL |
0.7 |
0.9 |
0 |
0 |
|
Triple-negative |
20 |
27 |
0 |
6.7 |
|
Incomplete or unknown |
8.8 |
9.1 |
7.4 |
0 |
Mutation status
The driver mutation status was available for 405 patients and showed that JAK2V617F was the predominant mutation in all MPN subtypes (Figure 1). Triple-negative (i.e., the absence of driver mutations in JAK2, CALR, and MPL) disease was more common in children, whereas mutated JAK2 was more common in adolescents/young adults (diagnosed before the age of 25 years). The median mutant allele burden was 22% for the whole cohort.
Notably, logistic regression analysis revealed that JAK2V617F mutation and hyperviscosity symptoms were risk factors for thrombosis risk.
Figure 1. Mutational landscape for each MPN subtype* .
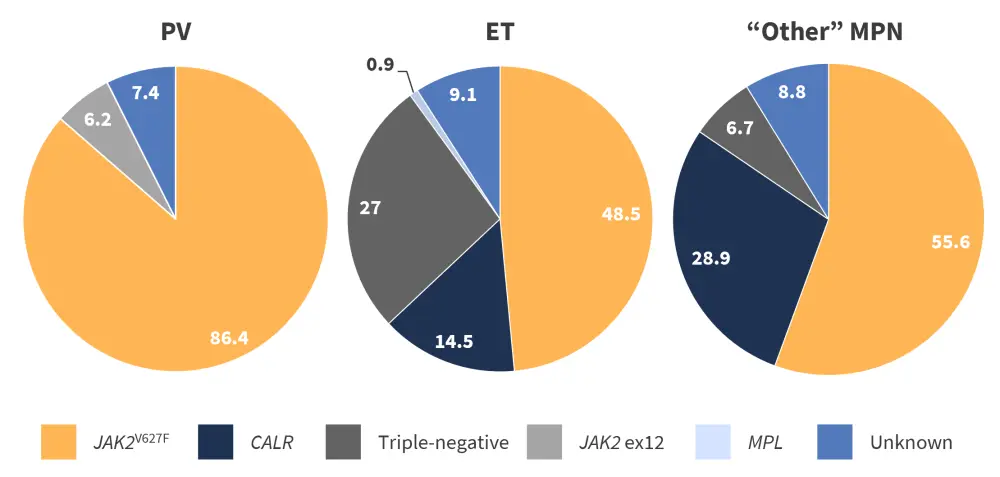
ET, essential thrombocythemia; MPN, myeloproliferative neoplasms; PV, polycythemia vera.
*Adapted from Sobas, et al.1
Treatment
During follow-up, 301 patients received ≥1 cytoreductive treatment, 333 patients received antiplatelet and anticoagulant therapy, and 47 did not receive any treatment. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) was performed in seven patients. The distribution of therapies across disease subgroups is shown in Figure 2.
Figure 2. Distribution of treatments across disease subtypes*
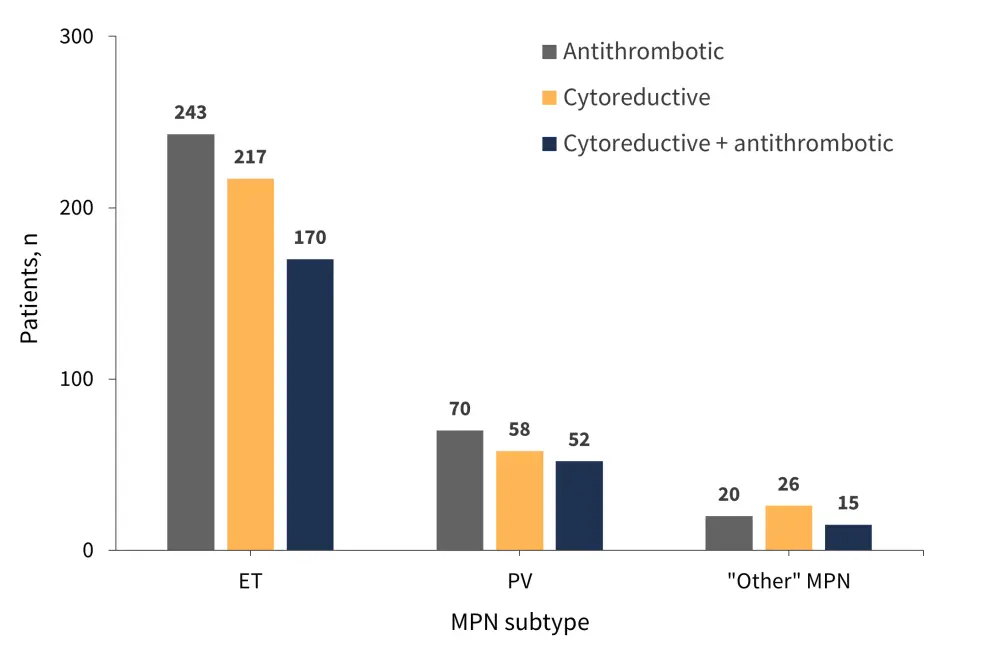
ET, essential thrombocythemia; MPN, myeloproliferative neoplasms; PV, polycythemia vera.
*Data from Sobas, et al.1
Thrombosis
A total of 49 patients had a history of thrombosis. Of these, 27 were patients with ET, 17 with polycythemia vera (PV), and five with “other” MPN. The 5-year incidence rate was 5.67% across the cohort, with a median time from diagnosis to first thrombotic event of 5 years. In comparison, the median time from first to second event was 4.4 years. One-third of patients experienced recurrent events, with venous events being more frequent than arterial. Perihepatic vein thrombosis was the most common, accounting for 47.6% of all venous events. Moreover, across the cohort, the presence of JAK2 mutation, along with hyperviscosity and thrombosis history, was a significant predictive factor of thrombosis-free survival.
Hemorrhage
A total of 25 patients had a prior history of hemorrhage; 19 of these patients had a diagnosis of ET. Overall, 44 patients experienced hemorrhagic events (1 event in each patient), which were more common in patients with PV and “other” MPN compared with ET (Figure 3). The overall thrombosis incidence rate was 1.04% patients/year, with a 5-year incidence rate of 4.37% across the whole cohort. The median time from diagnosis to first event was 4.7 years.
Univariate analysis identified several factors associated with a significant increase in risk of bleeding, including:
- Hyperviscosity (odds ratio [OR], 2.17; 1.07–4.37; p = 0.039)
- Splenomegaly (OR, 3.05; 1.50–6.20; p = 0.004)
- History of bleeding (OR, 11.05; 4.66–26.20; p = 0.000)
- Platelet count >1,500 g/L (OR, 2.61; 1.14–5.98; p = 0.031)
Figure 3. Frequency of new hemorrhage events by MPN subtype*
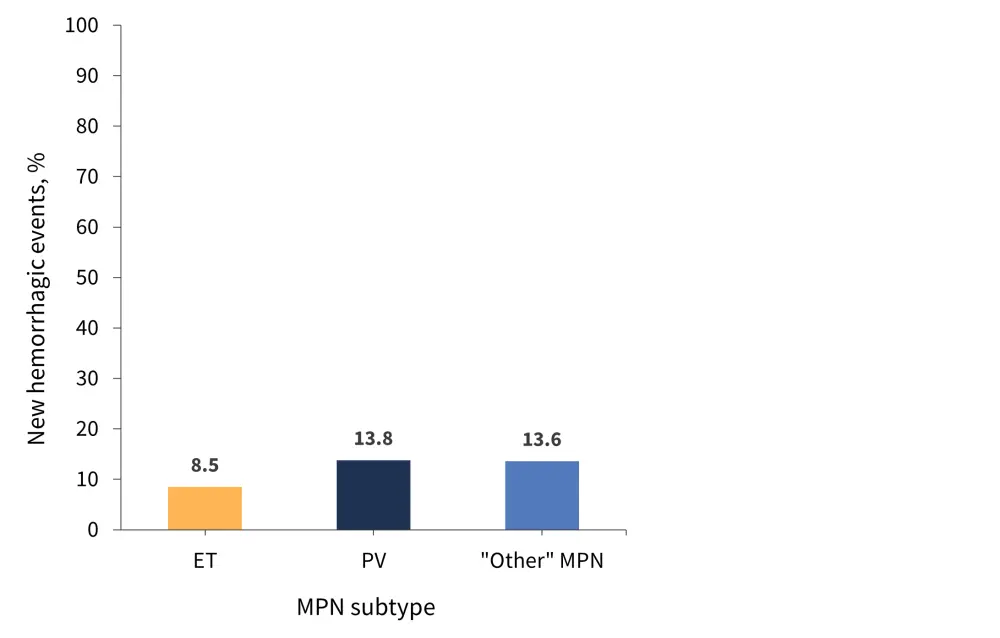
ET, essential thrombocythemia; MPN, myeloproliferative neoplasms; PV, polycythemia vera.
*Data from Sobas, et al.1
Phenotypic evolution
There were 48 phenotypic evolutions (10.9%) across the cohort, with 10% occurring in patients with ET and PV, and 15.6% in patients with “other” MPN. The global incidence rate was 1.13% patients/year. The most common transformation was evolution to secondary myelofibrosis, which had a 5-year incidence of 2.49% across the cohort. Transformation to PV occurred in 12 patients, all of whom harbored the JAK2V617F mutation. Palpable splenomegaly was noted to be the only significant risk factor for evolution.
Mortality
Eight deaths occurred throughout the study, with three of these occurring after allo-HSCT. Some causes of death included bleeding, leukemia, and solid cancer.
Conclusion
Sobas, et al. performed the largest real-world analysis of young patients with MPN to date, which highlighted a high disease burden, including high rates of thrombotic events and transformations. Limitations include the retrospective format and an underrepresentation of the total number of cases per country due to the EHA-SWG patient centers not being the exclusive care centers for MPN. There were also challenges in detecting splenomegaly and confirming mutational status. Despite this, the authors concluded that this is a reliable representation of MPN in young patients due to the rarity of young adults or adolescents presenting with MPN; however, further analysis should be performed in order to better manage risks and identify further unmet needs.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

