All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Understanding of real-world management patterns for myelofibrosis (MF) is elusive due to the diversity of treatment options. Considering the burden of MF-related symptoms, such as splenomegaly and anemia, it is important to understand whether current treatment initiation is time efficient; for example, if patients who are undergoing the ‘watch-and-wait’ approach may benefit from treatment instead, and whether optimal care is being given for the correct patients according to recognized international prognostic scoring systems.
The REALISM UK multicenter, noninterventional retrospective study investigated the early management strategies for MF in the UK, and was recently published by Mead et al. in Therapeutic Advances in Hematology.1 We summarize the key findings below.
Methodology
The study design, including primary and secondary endpoints, is depicted in Figure 1. A total of 15 National Health Service (NHS) hospitals participated in the data collection.
Figure 1. Study design*
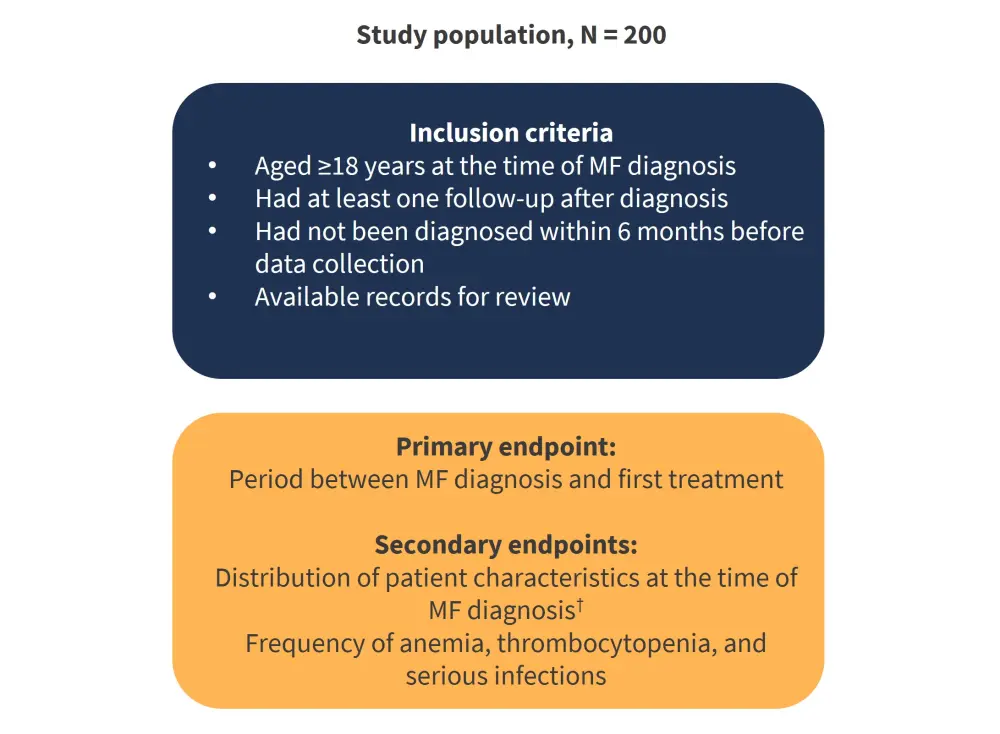
MF, myelofibrosis.
*Adapted from Mead et al.1
†Patient characteristics included demographics, diagnosis method, distribution of MF types, mutational status, MF symptoms, spleen size, IPSS prognostic score, and percentage of patients with recorded IPSS scores at diagnosis.
Results
Patient characteristics are shown in Table 1.
Table 1. Patient characteristics*
|
ET, essential thrombocytopenia; IPSS, International Prognostic Scoring System; JAK2, Janus kinase 2; MF, myelofibrosis; PV, polycythemia vera. |
|
|
Characteristic |
N = 200 |
|---|---|
|
Median age, years (range) |
69.7 (20.3–91.8) |
|
Female/male, % |
40.5/59.5 |
|
MF diagnosis, % |
|
|
Primary |
63.0 |
|
Secondary (post-ET) |
16.5 |
|
Secondary (post-PV) |
20.5 |
|
Positive for JAK2V617F mutation, % |
81.0 |
|
IPSS risk category, % |
|
|
Low-0 |
7.5 |
|
Intermediate-1 |
29.0 |
|
Intermediate-2 |
29.5 |
|
High ≥3 |
19.5 |
|
Unavailable |
14.5 |
Documentation of symptoms and prognostic scores
- Symptoms and prognostic scores were poorly documented overall, with clinical scores not recorded in 168 patients
- Scores were recorded for 11 patients with the International Prognostic Scoring System (IPSS), 12 patients with the Dynamic IPSS (DIPSS), 10 patients with the DIPSS-Plus, and three patients with other scoring systems
- Almost half (49%) of patients were classified as intermediate-2 or high-risk at diagnosis; however, it was noted that some scores were calculated as ‘0’ where data were unavailable, and so the actual proportion may be higher
- Total of 117 patients (58.5%) had symptoms prior to the myeloproliferative neoplasms (MPN) diagnosis, and only 20 patients had an assessment using a documented MPN-Symptom Assessment Form Total Symptom Score (MPN-SAF TSS)
- Most patients were diagnosed through patient-reported symptoms and laboratory tests.
- Splenomegaly and anemia were the two most reported symptoms at diagnosis (47% and 44%, respectively) followed by unexplained tiredness, unintended weight loss, and abnormal sweats.
- Overall, 57% of patients were recorded to have no constitutional symptoms at diagnosis
First-line treatment
- Active treatment was given at diagnosis in 46.5% of patients.
- The other 53.5% of patients were on a ‘watch-and-wait’ strategy for their initial core management.
- The median time to active treatment, stratified by IPSS risk score is summarized in Table 2.
Table 2. Median time to active treatment from diagnosis by IPSS risk score*
|
IPSS, International Prognostic Scoring System. |
|
|
IPSS risk score |
Median time to treatment, days (range) |
|---|---|
|
Overall (n = 171) |
46 (0–342) |
|
Low-0 (n = 15) |
153 (0–667) |
|
Intermediate-1 (n = 58) |
89.5 (0–473) |
|
Intermediate-2 (n = 59) |
0 (0–251) |
|
High ≥3 (n = 39) |
0 (0–216) |
- Overall, patients with high-risk disease were given active treatment sooner
- However, some patients with high-risk classification were on a ‘watch-and-wait’ treatment strategy
- ‘Watch-and-wait’, ruxolitinib, and hydroxycarbamide (aka hydroxyurea) were the most common first-line approaches (Figure 2).
Figure 2. First-line treatment by IPSS risk score*
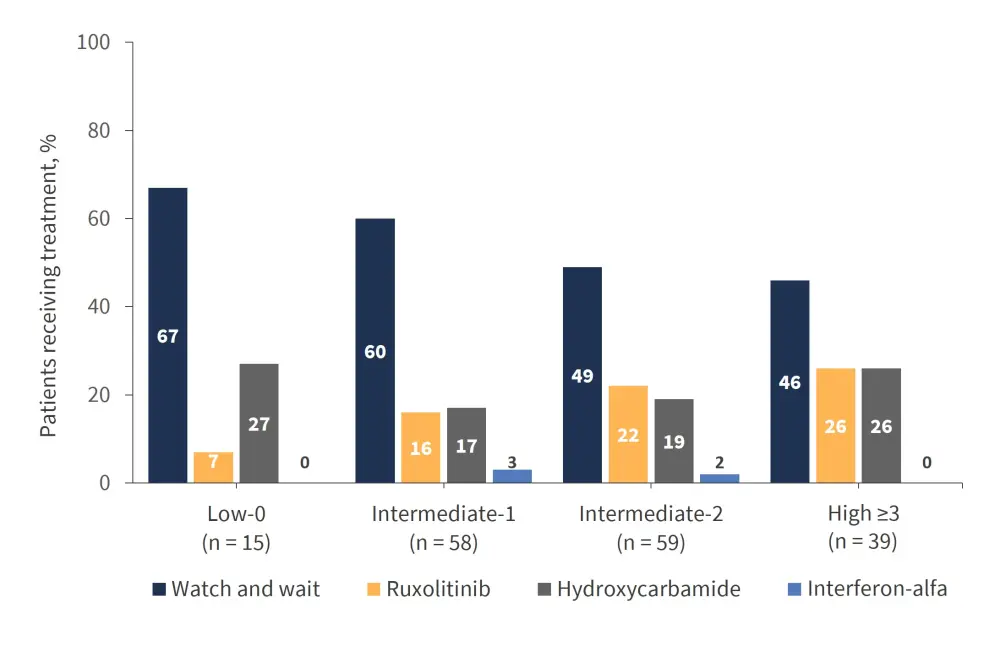
IPSS, International Prognostic Scoring System.
*Adapted from Mead et al.1
- Notably, interferon-alfa was rarely used
- The proportion of patients undergoing the ‘watch-and-wait’ management decreased, and the percentage of patients receiving ruxolitinib increased with an increasing risk score.
- A total of 134 courses of the ‘watch-and-wait’ management strategy were recorded, and 81 persisted for ≥6 months
- A total of 111 courses of ruxolitinib were recorded, with 81 persisting for ≥6 months
- A total of 68 courses of hydroxycarbamide treatment were recorded, with 44 persisting ≥6 months
- Allogeneic hematopoietic stem cell transplantation (allo-HSCT) was given to only 10 patients (5%), none of whom received this as a first-line treatment
- A total of 39 patients were <70 years old at diagnosis with an IPSS score of intermediate-2 or high-risk, and three underwent allo-HSCT (8%) which is slightly higher than the overall population
- Median age for allo-HSCT was 59.5 years
- As well as the IPSS risk score, the ‘watch-and-wait’ management decreased in incidence in more recent years (Figure 3)
Figure 3. First-line treatment strategies by year*
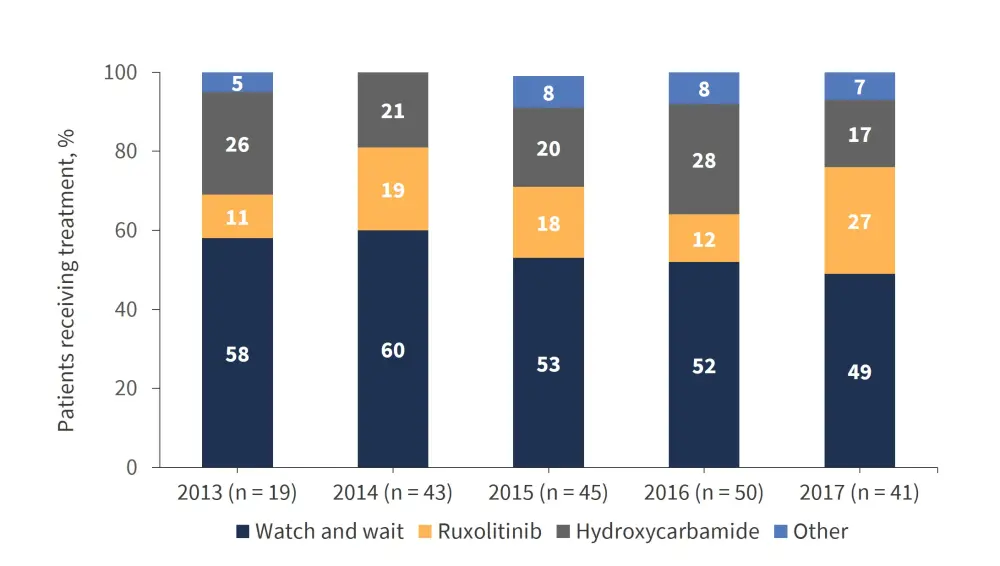
*Figure from Mead et al.1
Treatment duration
The shortest median duration of treatment (interquartile range [IQR]) was observed for ruxolitinib (541.5 days [313.5–998.8]), then hydroxycarbamide (608.0 days [407.5–988.5]), and finally, ‘watch-and-wait’ (619.0 days [392.0–973.0]).
Causes of death
- A total of 47 patients died while receiving MF management
- Of these deaths, 63.8% were MF-related
- The causes of death are summarized in Figure 4.
Figure 4. Causes of death*
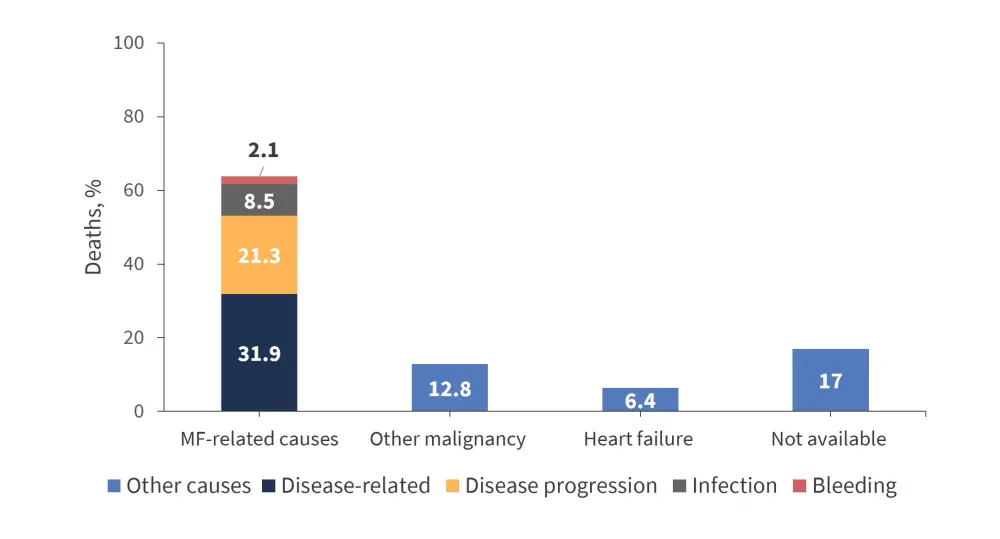
MF, myelofibrosis.
*Adapted from Mead et al1
Conclusion
Retrospective, real-world data from the REALISM UK study demonstrate a lack of substantial recording of patient-reported symptoms and diagnostic criteria which are important for deciding treatment. Although in decline in recent years, almost half of patients were reported to have ‘watch-and-wait’ treatment, which was sometimes given for patients classified as high risk. These data demonstrate a need for earlier treatment intervention in the UK. Hydroxycarbamide remains a common approach, though less effective than ruxolitinib, indicating that patients with a high symptom burden were treated with options that may not improve disease symptoms. The discontinuation rates with hydroxycarbamide and ruxolitinib were high, highlighting the need for more understanding of reasons behind this, and a guidance on when to discontinue treatment.
Expert Opinion
In this Novartis sponsored real world observational note review we focussed upon the pathway from diagnosis to treatment for myelofibrosis in the UK. We found that both symptoms and prognostic scores at diagnosis were poorly documented. Time to first treatment was shorter for patients with higher risk disease and only 5% of patients underwent stem cell transplant. Other important insights included that 35% of patients required blood transfusion.
 Claire Harrison
Claire HarrisonReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

