All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Results from a phase III study demonstrate benefit with mepolizumab over placebo in patients with hypereosinophilic syndrome
Hypereosinophilic syndromes (HES) are a group of rare myeloproliferative neoplasms (MPN) characterized by elevated eosinophil levels. Abnormally high eosinophil counts eventually contribute to organ damage and, if left untreated, can become life threatening. Interleukin-5 (IL-5) is a major growth factor for eosinophils, and the anti-IL-5 monoclonal antibody, mepolizumab, has been approved for the treatment of several eosinophilic diseases. The question remains whether mepolizumab could be effective in the HES setting.
Results from the phase III study evaluating the efficacy and safety of mepolizumab vs placebo in patients with HES, were presented by Florence Roufosse1 during the virtual edition of the 25th European Hematology Association (EHA) Annual Congress, and the data are summarized here.
Study design
- Phase III, randomized, double blind, multicenter, placebo-controlled trial conducted as a parallel group study
- Primary endpoint: Proportion of patients who experienced a flare in the 32-week study period (defined as either, a clinical manifestation of HES requiring an increase of ≥ 10 mg prednisolone or similar for 5 days, or an increase/the addition of cytotoxic or immunosuppressive therapy, or having received more than two courses of oral corticosteroids during treatment)
- Secondary endpoints: Time to first flare, annual rate of flares and the proportion of patients who experienced a flare in weeks 20–32 of the study period
Patient eligibility
- Patients were aged ≥ 12 years with a diagnosis of HES* ≥ 6 months prior to the trial onset
- Patients received HES therapy ≥ 4 weeks prior to the baseline visit
- Patients had uncontrollable HES defined as
- ≥ two flares within 12 months of trial onset
- blood eosinophil count ≥ 1,000 cells/µL
*FIP1L1-PDGFRA-negative HES
Treatment
- Eligible patients (N = 108) underwent screening followed by a 32-week treatment period during which they were randomized to receive existing HES therapy plus
- 300 mg subcutaneous mepolizumab (n = 54) or placebo (n = 54)
Results
Patient characteristics
- Patient characteristics at the time of enrollment are presented in Table 1
Table 1. Baseline patient characteristics1
|
BMI, body mass index; HES, hypereosinophilic syndromes; OCS, oral corticosteroids |
||
|
Characteristic |
Placebo (n = 54) |
Mepolizumab (n = 54) |
|---|---|---|
|
Median age, years (range) |
45.00 (15.00–80.00) |
47.00 (12.00–82.00) |
|
Female sex, % |
50.00 |
56.00 |
|
Mean HES duration, years |
5.70 |
5.50 |
|
Mean BMI, kg/m2 |
26.20 |
26.38 |
|
Geometric mean blood eosinophil count cells/µL |
1350.00 |
1460.00 |
|
Treatment, % |
|
|
|
OCS |
70.00 |
74.00 |
|
Cytotoxic/immunosuppressive therapy |
17.00 |
26.00 |
Efficacy
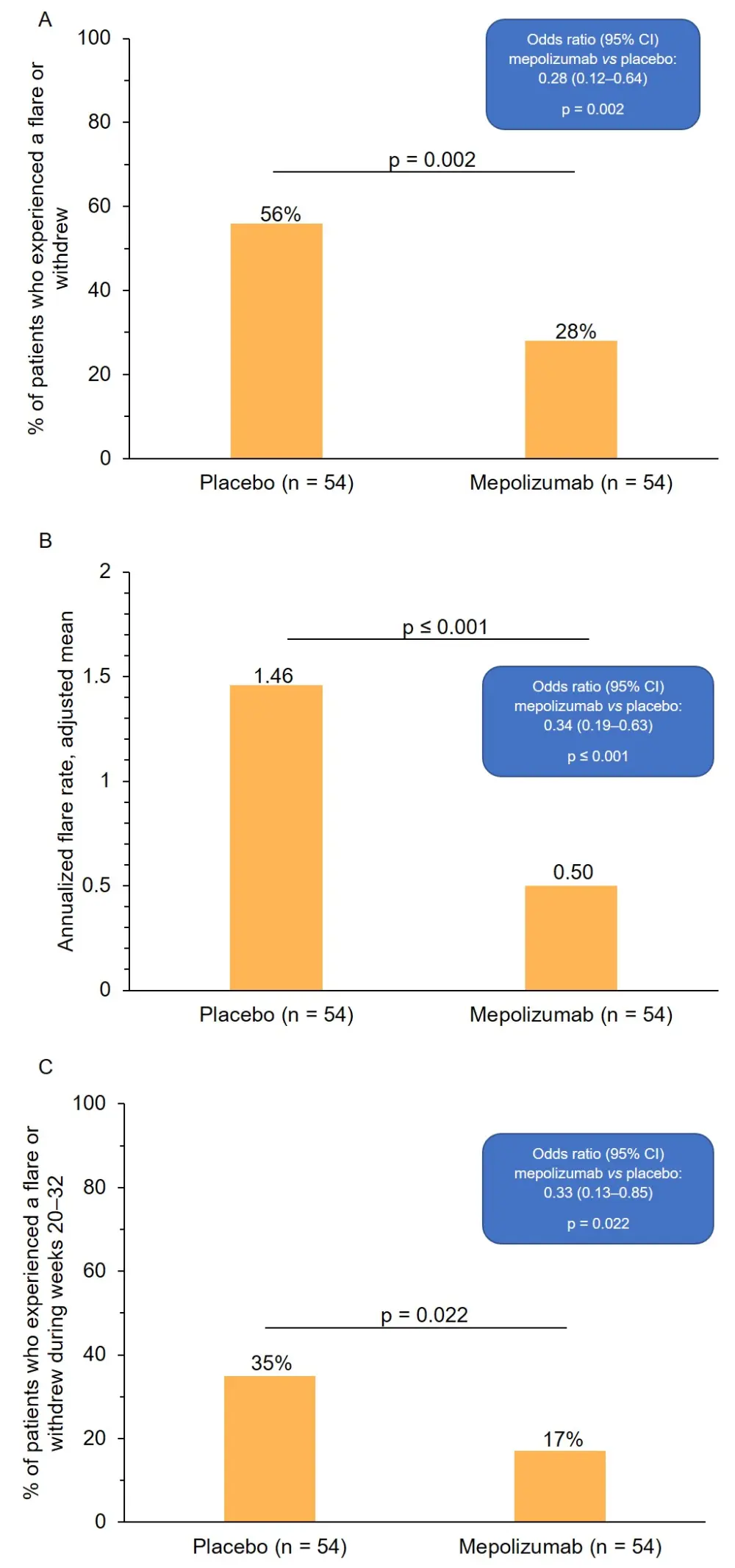
- A 50% reduction in the proportion of patients who experienced a flare or withdrew from the study was observed in the mepolizumab vs placebo groups (Figure 1A)
- A 66% reduction in the annualized rate of flares was observed in the mepolizumab vs placebo groups (Figure 1B)
- A 53% reduction in the proportion of patients who experienced a flare or withdrew from the study in the final 12 weeks of the study period was observed in the mepolizumab vs placebo groups (Figure 1C)
- Mepolizumab induced a significant reduction in blood eosinophil levels, which plateaued after three doses, when compared with placebo
Figure 1. Patient responses to mepolizumab. A, proportion of patients who experienced a flare or withdrew from the study across the 32-week study period. B, annualized rate of flare. C, proportion of patients who experienced a flare or withdrew from the study during weeks 20–32 of the study period. CI, confidence interval
Safety
- Frequencies of adverse events (AEs) were similar in the placebo and mepolizumab arms
- No serious AEs were considered to be treatment related
- The most frequently observed on-treatment AEs are presented in Table 2
Table 2. Most common on-treatment AEs reported in ≥ 10% of patients in any group1
|
AE, adverse event |
||
|
AE, % |
Placebo (n = 54) |
Mepolizumab (n = 54) |
|---|---|---|
|
Bronchitis |
19 |
15 |
|
Diarrhea |
13 |
9 |
|
Headache |
13 |
13 |
|
Nasopharyngitis |
13 |
13 |
|
Pain in extremity |
4 |
11 |
|
Pruritis |
13 |
7 |
|
Rhinitis |
11 |
9 |
|
Upper respiratory tract infection |
4 |
15 |
Conclusions
Data from this study have demonstrated that, when added to patient standard of care, mepolizumab significantly reduces the frequency of flares and blood eosinophil levels when compared with placebo, and no new safety concerns were associated with mepolizumab. Professor Roufosse concluded that the findings from this study suggest that mepolizumab may offer patients with FIP1L1-PDGFRA-negative HES an effective option for management, which is currently an area of unmet clinical need.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

