All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
The influence of MPN on SARS-COV-2 infection, mortality, and thrombotic events
Featured:
Hematologic malignancies are an underlying risk factor for SARS-COV-2 infection and mortality. More specifically, patients with myeloproliferative neoplasms (MPN) are at increased risk of thrombotic events, which is likely compounded by SARS-COV-2 infection, a factor also associated with arterial and venous thromboembolism (VTE) in the general population.1
MPN Hub steering committee members Tiziano Barbui and Valerio De Stefano recently published a review in Current hematologic malignancy reports, summarizing the associations of MPN phenotypes and other factors with SARS-COV-2 infection, mortality, and thrombotic events. They also used data from the general population to guide recommendations for antithrombotic prophylaxis during SARS-COV-2 infection. The key findings from this review are summarized below.1
Incidence and mortality of patients with MPN and SARS-COV-2 infection
Data analyzed in this review were primarily from a European LeukemiaNet (ELN) study, previously summarized on our Hub. Here, the epidemiology of SARS-COV-2 was reported in different MPN phenotypes between February and June 2020.
Out of 175 patients with SARS-COV-2 infection, the majority (44%) had primary myelofibrosis (PMF) and the mortality rate of patients with MPN was two to three times higher than the general population with SARS-COV-2. Univariate analysis revealed myelofibrosis was associated with increased risk of mortality (48%) compared with essential thrombocytopenia (25%), and polycythemia vera (19%). In a multivariate analysis, abrupt withdrawal of ruxolitinib was associated with increased mortality risk; the American Society of Hematology advise tapering ruxolitinib cautiously.
Excess mortality risk with MPN in SARS-COV-2 infection was also noted in the ASH Research Collaborative SARS-COV-2 Registry. Hospitalized patients with MPN had a mortality rate of 34%; three-fold higher than in the general population.
Thrombosis in patients with MPN and SARS-COV-2
Analysis of data from 162 patients (ET, n = 48; PV, n = 42; PMF, n = 56; pre-PMF, n = 16) in the ELN study revealed:
- Overall, 15 cases of thrombosis were reported, of which 12 were VTEs.
- Rate of VTE was highest in patients with ET (16.7%) compared with PV (4.8%) or MF (3.6%; p = 0.031).
- Risk of thrombosis was higher in patients treated in intensive care units (ICU) (18.4%) compared to patients at home (1.0%) or on the regular ward (2.8%).
- From multivariate analysis including the above factors, transfer to ICU (hazard ratio [HR], 3.73; p = 0.029), neutrophil/lymphocyte ratio (HR, 1.1; p = 0.001), and ET phenotype (HR, 4.37; p = 0.006) produced significant risk associations with thrombosis.
- The authors highlighted the difficulty in comparing these results with the general population, given that the rate of thrombosis varies greatly depending on screening for its presence.
- Pneumonia and multiorgan failure were the most common causes of mortality, particularly in ET, so the association of micro-thrombotic events in organs could not be ruled out.
- The survival probability of patients with ET (53%) was much lower than patients with MF/PV (75%; p = 0.05), suggesting micro-thrombosis as a cause of these fatal events.
Bleeding in MPN phenotypes
Major bleeding occurred in seven of the 162 (4.3%) patients with MPN and SARS-COV-2, higher than in the general population (2.3%). This was more common in MF, resulting in a high rate of blood transfusions.
Bleeding events were reported at a later stage than thrombotic events (~two weeks after SARS-COV-2 diagnosis). Coagulopathy or severe thrombocytopenia was associated with bleeding in four cases, indicating a need for lower doses of anticoagulant therapies, including low molecular weight heparin (LMWH).
Anticoagulant prophylaxis in patients with MPN and SARS-COV-2
Of the 162 patients in the ELN study receiving heparin prophylaxis, 19 experienced 22 thrombo-haemorrhagic events either at home (1/40, 2.5%), during hospitalization (13/105, 12.4%), or in ICU (5/17, 29.4%). Furthermore, ten of the 12 patients who experienced VTE received LMWH, of which half were on low/intermediate doses and half were on therapeutic doses. Given that the mortality rate of patients with thrombosis was 50% (7/14), the authors recommended that all patients with MPN hospitalized with SARS-COV-2 should receive prophylactic doses of LMWH.
This advice is aligned with that for the general population, as only the ELN study reported thrombotic events in patients with MPN. A risk stratified approach was thought to be most beneficial. Those with more severe infection requiring hospitalization will have LMWH at standard dose added to standard antiplatelet agent regimens for primary prophylaxis. For secondary prophylaxis or atrial fibrillation, anti-vitamin k antagonists or direct oral anticoagulants should be replaced completely with LMWH at therapeutic doses (Figure 1).
Figure 1. A flow chart for antithrombotic prophylaxis dependent on the severity of SARS-CoV-2 infection*
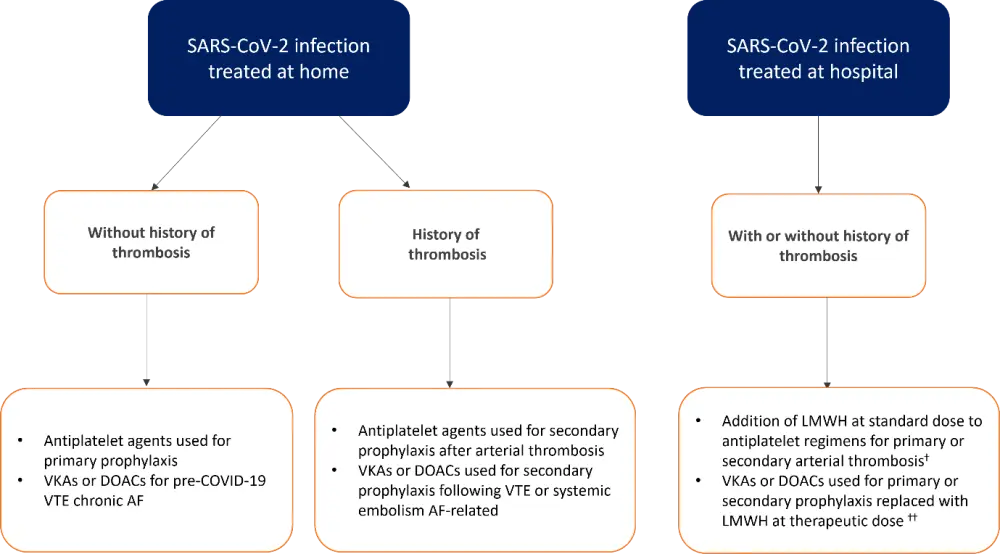
AF, atrial fibrillation; DOAC, direct oral anticoagulant; VKA, anti-vitamin K antagonist; VTE, venous thromboembolism.
*Adapted from Barbui, et al.1
†Standard dose of LMWH, enoxaparin at 40mg/d.
††Therapeutic dose of LMWH, enoxaparin at 100mg/kg, twice daily.
Conclusion
Overall, this review confirmed the associated risk of MPN with SARS-COV-2 infection and related mortality. Furthermore, PMF and abrupt discontinuation of ruxolitinib were identified as independent risk factors for mortality. In addition, ET was associated with greater risk of VTE, and the importance of other micro-thrombotic events that contribute to multiorgan failure was also raised. Although there are limited data, thrombotic prophylaxis during SARS-COV-2 infection in patients with MPN should align with the recommendations for the general population; notably, replacement or addition of heparin was recommended in higher risk infection settings.
Tiziano Barbui has provided further insight into these findings and the aims for further data collection in subsequent waves of SARS-COV-2, which can be read below.
Expert Opinion
In early March 2020, Bergamo (population of 1,200,000) was the epicenter of the coronavirus pandemic and in that month had 4,500 deaths from COVID-19. I had the infection, together with my wife and my daughter, who is a hematologist at the Bergamo hospital. Fortunately, it was not necessary to be hospitalized and in these days I launched, together with my collaborators from the Research Foundation -FROM, a European study approved by the European LeukemiaNet (ELN) to understand if patients with myeloproliferative diseases could be more vulnerable to infection.
The major blood centers of Italy, Germany, France, England, Spain, Croatia, and Poland participated. The short period to obtain approval from the national ethics committees was incredible, so that in a few months we collected the information we had requested in a specific pre-established protocol. In June 2020 we had electronically registered ~200 patients, and we were ready for statistical analysis to be performed at our institution. We found that patients with myelofibrosis were more prevalent than patients with polycythemia or thrombocythemia, and that their mortality was higher due to both the severity of the disease and the discontinuation, during the acute phase of the infection, of the drug ruxolitinib.
This finding was published, communicated to the American Society of Hematology (ASH), and the guidelines promptly recommended that the drug ruxolitinib should not be withdrawn during acute infection. Therefore, the practical implication that clinical research in a state of great emergency has had was extraordinary. The result was similar concerning the elevated occurrence of thrombosis found in thrombocythemia, affecting survival in a great proportion of these cases.
At the request of the MPN scientific community, we have transformed this initial data collection into a register that is still active. This is allowing us to compare the second and subsequent waves of SARS-COV-2 infection with the first. We will present the results from >400 patients with MPN and COVID at the next ASH congress in Atlanta. We will show how the early diagnosis of the infection and the current therapy have made it possible to substantially reduce the hospitalization of patients and their mortality. A significant role for vaccines is emerging and, fortunately, we have not at the moment collected any record of severe vaccine reactions in our cases.
I would like to emphasize the collaboration that has led to these results and the importance of research networks in rare diseases such as MPN.
 Tiziano Barbui
Tiziano BarbuiReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

