All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Updates of IMG-7289 (bomedemstat) in treating myelofibrosis and essential thrombocythemia
Lysine-specific demethylase-1 (LSD1) is a histone H3K4 demethylase that is required for the regulation of the proliferation of blood stem cells, and the differentiation of progenitor cells into mature megakaryocytes. It is overexpressed in myeloproliferative neoplasms (MPN) and LSD1 inhibition has been associated with the loss of self-renewal of malignant myeloid cells.
IMG-7289 (bomedemstat) is an orally active LSD1 inhibitor which is known to have favorable impact on myelofibrosis (MF) symptoms. Recently, Gill et al.1 and Ross et al.2 presented updated results at the 26th Congress of the European Hematology Association (EHA2021), for two ongoing phase II studies in patients with MF (IMG-7289-CTP-102; NCT03136185) and essential thrombocythemia (ET) (IMG-7289-CTP-201; NCT04254978), respectively.
Updated results from patients with MF1
Study design
This is a multinational, open-label, 24-week phase II study in patients with MF. Further information—the study design including primary endpoints, eligibility criteria, and previous results from a 12-week analysis—can be found here.
The starting dose was 0.6 mg/kg once daily given orally. Dose adjustments were made individually to achieve a platelet count of 50−75 × 109/L.
Patients
A total of 86 patients were enrolled; 62% completed at least 12 weeks of study, and 49% are still on study (Figure 1).
Figure 1. Patient enrollment according to the most recent updates of the study*
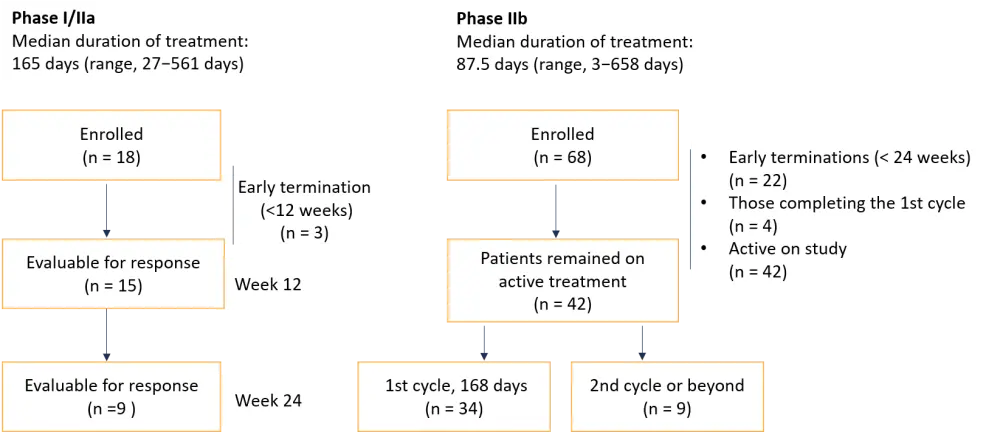
*Adapted from Gill et al.1
The reasons for early termination before Week 24 included adverse events (AEs) (related or unrelated to treatment), investigator decision, disease progression, limited clinical benefit, and other reasons.
Results
Baseline characteristics are similar to those included in the 12-week analysis as published previously.
Efficacy
- There was an improvement in symptoms reduction from Week 12 to Week 24 where more patients had reduction in symptoms by Week 24. Of all patients with total symptom score (TSS) >20 (n = 16), 94% recorded a reduction in TSS and 31% had a decrease of ≥50% in TSS at Week 24.
- Of all patients evaluable for spleen volume reduction (SVR) at 24 weeks (n = 18), 89% had a reduction in spleen volume from baseline. One patient had a decrease (≥35%) at 24 weeks.
- Also, 26% of evaluable patients had an improvement in the fibrosis score by one grade while 43% were stable.
- Elevated serum lactate dehydrogenase improved or resolved in 88% of patients.
- Changes in hemoglobin (Hb) in transfusion-independent patients at Week 12 (n = 24) were as follows:
- 83% had stable/improved Hb
- 54% had an increase of ≥1.0 g/dL
- 29% had stable Hb (∆ <±1.0 g/dL)
- Transfusion treatment was recorded in 28 patients; 14 patients were evaluable for changes in transfusion burden:
- Eight patients were stable (∆ <±1 unit/month change over predose)
- Four patients were worsening (≥1unit/month increase over predose)
- One improved (≥1 unit/month decrease over predose)
- One became transfusion independent
- A strong correlation was observed between improved Hb and reduction in TSS; however, not with SVR.
- In 34 patients, mutant allele frequencies (MAFs) in driver and high-molecular risk (HMR) mutations were:
- reduced in 44% of patients
- stable in 47%
- increased in 9% of patients
- All driver mutations showed sensitivity to IMG-7289; ASXL1 clones were subject to purifying selection by treatment.
- Increased baseline blasts (n = 24) improved or resolved in 71% of patients. There has been no progression to acute myeloid leukemia (AML) at up to 660 days.
Safety
- 398 of the 1,086 AEs were considered as treatment related.
- The most common treatment-related nonhematologic AE was dysgeusia (n = 21; 24%).
- There were 10 serious AEs (Grade 2, n = 3; Grade 3, n = 7), which were considered related to IMG-7289 including vertigo, splenomegaly, headache, cardiac failure, rectal hemorrhage, gastrointestinal hemorrhage, pyoderma gangrenosum, anemia, nausea, and hematoma.
- No treatment-related deaths were reported.
Conclusion
Patients with MF had an improvement in symptom scores, spleen volume, and anemia. A decrease in MAFs was seen in 44% of evaluable patients including driver and HMR mutations, such as ASXL1. No new mutations or transformation to AML were observed in patients at high-risk of progression. The reduction in MAFs and suppression of new mutations observed suggest that bomedemstat may be a disease-modifying therapy with respect to AML.
Results from patients with ET2
This is an ongoing, multinational, open-label, 24-week phase IIb study in patients who had the following characteristics:
- were diagnosed with ET
- high-risk classification
- failed at least one standard therapy
- had platelets >450 × 109/L
- had peripheral blasts <1%
- with a fibrosis score <2 per protocol criteria
- had Hb >10 g/dL
Treatment plan
- Patients were given IMG-7289 once/day, orally at a 0.6 mg/kg starting dose.
- Dose adjustment were made individually for each patient in both phases to achieve a platelet count of 200−400 × 109/L in patients with ET.
Study endpoints
Key objectives of the CTP-201 study in patients with ET2
- Primary endpoints
-
- Safety and tolerability
- Platelet count reduction (≤400 × 109/L) in the absence of thromboembolic events
- Exploratory endpoints
-
- Durability of platelet and white blood cell (WBC) count reduction
- Changes in cytokine profiles
- Symptom reduction (myeloproliferative neoplasm symptom assessment form [MPN-SAF] total symptoms score [TSS])
- Changes in MAFs
Patients
A flow diagram showing patient enrollment is depicted in Figure 2.
Figure 2. Flow diagram of patient enrollment in the beginning of the study and active participants according to the most recent updates of the study*
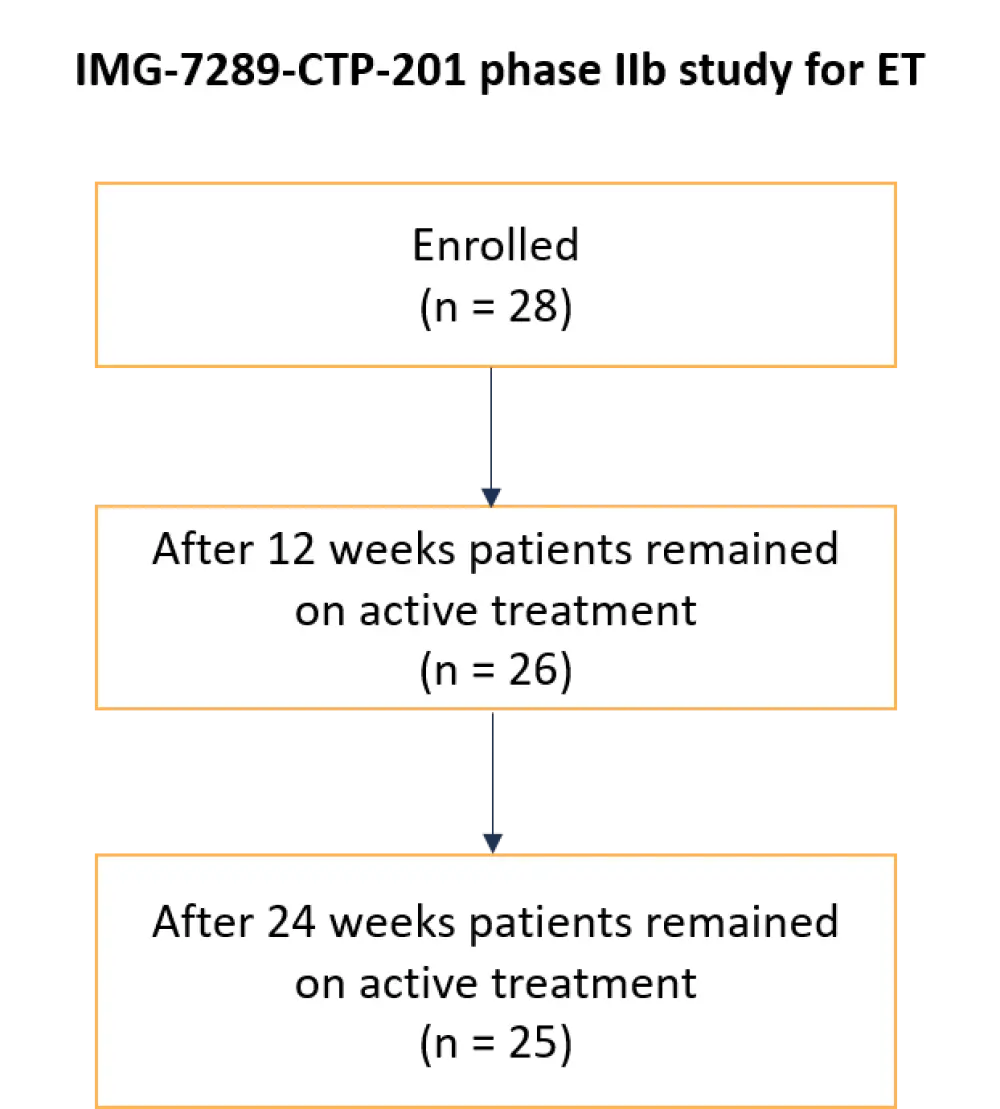
ET, essential thrombocythemia.
*Adapted from Ross et al.2
Results
The results summarized below were obtained at Week 12. Baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics for patients with ET*
|
CALR, calreticulin gene; ET, essential thrombocythemia; Hb, Hemoglobin; JAK2, Janus kinase 2 gene; TSS, total symptom score; WBC, white blood cells. |
|
|
Characteristic |
N = 28 |
|---|---|
|
Median age, years (range) |
67 (42−84) |
|
Male, % |
36 |
|
Mean blood counts |
|
|
WBC |
9.9 × 109/L |
|
Hb |
13 g/dL |
|
Platelets |
892 × 109/L |
|
Mutation (n = 20), % |
|
|
JAK2 |
50 |
|
CALR |
40 |
|
Triple-negative |
10 |
|
≥1 myeloid disease-associated mutation |
30 |
|
Median symptom score |
30 (range, 11−74) |
|
Patients with TSS >10, n |
18 |
|
Most recent therapy prior to bomedemstat, % |
|
|
Hydroxyurea |
78 |
|
Anagrelide |
7 |
|
Interferon |
7 |
|
Ruxolitinib |
4 |
|
Other |
4 |
Efficacy
- 83% of patients dosed for >6 weeks achieved a platelet count of <400 × 109/L (n = 12); a mean reduction of 547 × 109/L was recorded at Week 12 (n = 10).
- At Week 12 (n = 10), a mean WBC reduction of 3.3 × 109/L was observed. WBC fell to normal (<10) in 80% of patients who previously had elevated WBCs.
- No neutropenia was observed.
- All patients had stable Hb values.
- Patients (n = 6) who had a TSS score of >10 at Day 1 observed a decrease in TSS (83%) at 12 weeks.
Safety
Seventy-eight of the 160 AEs reported by 19 patients were considered treatment related.
- Most common treatment-related nonhematologic AEs were dysgeusia and diarrhea (four each).
- One serious AE of lung infection was considered unrelated to IMG-7289.
Conclusion
Overall, for ET patients, IMG-7289 was considered as safe. Improvement on platelet and WBC counts was observed with a stable Hb. There was also a symptomatic improvement for patients with significant MPN symptoms. There was no obvious relationship among driver mutations, starting platelet counts, final optimal dose, or response rates. Data about changes in MAFs are expected to be known soon as patients reach 24 weeks.
Overall conclusion
IMG-7289 (bomedemstat) is a generally safe and well tolerated therapeutic option in patients with MF and ET and has shown clinical activity as a monotherapy. Another clinical trial investigating IMG-7289 in patients with polycythemia vera (PV) is currently enrolling patients (NCT04262141).
Cumulative safety experience from 159 patients with AML, myelodysplastic syndromes (MDS), MF, and ET have demonstrated that there are no dose-limiting toxicities to a maximum of 6 mg/kg daily dose. There were no safety signals in 4 years with IMG-7289. Furthermore, no genotoxicity or mutagenicity have been observed.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

