All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
5-year results from the PROUD-PV and CONTINUATION-PV studies
Ropeginterferon alfa-2b is a novel pegylated recombinant interferon, approved in the EU as monotherapy for the treatment of adults with polycythemia vera (PV) without symptomatic splenomegaly.
The PROUD-PV study is the first randomized, controlled, phase III study comparing hydroxyurea to ropeginterferon alfa-2b for the treatment of PV. Previously, the MPN Hub covered the final results of the PROUD-PV study, and the interim data from the 5-year planned extension study, CONTINUATION-PV, at 36 months. In addition, a subanalysis of the PROUD-PV trial according to age was reported in a recently published article.
During the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, Heinz Gisslinger presented the 60-month interim analysis from the PROUD-PV/CONTINUATION-PV studies; here we report a summary of this talk.
60-month interim analysis
This analysis included all patients enrolled in the CONTINUATION-PV (NCT02218047) study:
- 89.6% of patients (n = 95) treated with ropeginterferon alfa-2b in the PROUD-PV study entered in the CONTINUATION-PV extension study, and 73.7% of these patients (n = 70) continued their treatment until Month 60
- Similarly, 68.5% of patients (n = 76) treated with hydroxyurea in the PROUD-PV study enrolled in the CONTINUATION-PV extension study to receive best available treatment, where 88% of these were treated with hydroxyurea and 75% (n = 57) were treated up to Month 60
Both hydroxyurea-naïve and pre-treated patients were included, and pre-treated patients constituted 32.3% of the ropeginterferon alfa-2b arm vs 32.9% of the control arm.
The median dose of ropeginterferon alfa-2b per treatment cycle (4 weeks) in the fifth year was 499 μg, compared with > 700 μg in Years 1 and 2. During the first year of treatment, the drug was administered every 2 weeks, but eligible patients were later allowed to switch to administration every 3 or 4 weeks.
Efficacy
Complete hematologic response
Complete hematologic response (CHR, defined as hematocrit [HCT] < 45% and no phlebotomy for at least 3 months, platelets < 400 × 109/L, and white blood cells < 10 × 109/L) is reported in Table 1.
Table 1. CHR up to Month 601
|
CI, confidence interval; CHR, complete hematologic response. |
||||
|
Study month |
Ropeginterferon alfa-2b (n = 95) |
Control (n = 76) |
p value |
Rate ratio |
|---|---|---|---|---|
|
Month 12 |
59/95 (62.1) |
57/76 (75.0) |
0.1303 |
0.85 (0.70─1.05) |
|
Month 24 |
67/95 (70.5) |
33/67 (49.3) |
0.0129 |
1.41 (1.07─1.84) |
|
Month 36 |
67/95 (70.5) |
38/74 (51.4) |
0.0104 |
1.39 (1.08─1.78) |
|
Month 48 |
57/94 (60.6) |
34/76 (44.7) |
0.0275 |
1.39 (1.04─1.86) |
|
Month 60 |
53/95 (55.8) |
33/75 (44.0) |
0.0974 |
1.30 (0.95─1.77) |
At Month 60, the main reasons for not meeting CHR were premature discontinuation (in 25 patients [59.5%] vs 17 [40.5%] for the ropeginterferon alfa-2b arm vs the control arm) and elevated HCT (in 12 patients [28.6%] vs 9 [21.4%] for the ropeginterferon alfa-2b arm vs the control arm). Of the prematurely discontinued patients, the responses at last visit were:
- Ropeginterferon alfa-2b arm (n = 25), 68% CHR vs 32% no CHR
- Control arm (n = 17), 35% CHR vs 65% no CHR
The CHR with last observation carried forward (LOCF) is reported in Table 2. In this case, a statistically significant difference in responses between the ropeginterferon alfa-2b arm and the control arm was observed which deepened over time.
Table 2. CHR with LOCF1
|
CI, confidence interval; CHR, complete hematologic recovery; LOCF, last observation carried forward. |
||||
|
Study month |
Ropeginterferon alfa-2b (n = 95) |
Control (n = 76) |
p value |
Rate ratio |
|---|---|---|---|---|
|
Month 12 |
59 (62.1) |
57 (75.0) |
0.1 |
0.85 (0.70─1.04) |
|
Month 24 |
70 (73.7) |
43 (56.6) |
0.04 |
1.27 (1.02─1.60) |
|
Month 36 |
73 (76.8) |
41 (54.0) |
0.003 |
1.43 (1.13─1.81) |
|
Month 48 |
68 (71.6) |
38 (50.0) |
0.004 |
1.46 (1.13─1.89) |
|
Month 60 |
69 (72.6) |
40 (52.6) |
0.004 |
1.43 (1.12─1.81) |
Patients phlebotomy-free at 5 years of treatment reached 81.8% in the ropeginterferon alfa-2b arm and 63.2% in the control arm (p = 0.01).
Allele burden
The median JAK2 V617F allele burden is reported in Table 3. After Month 24, there was a significant reduction in JAK2 V617F allele burden observed in the ropeginterferon alfa-2b arm in comparison with the control arm. The ropeginterferon alfa-2b-induced suppression of allele burden continued up to Month 60, while allele burden was back to baseline in the control group.
Table 3. JAK2 V617F allele burden (using LOCF)1
|
CI, confidence interval; LOCF, last observation carried forward. |
||||
|
Study month |
Ropeginterferon alfa-2b (n = 95) |
Control (n = 76) |
p value |
Rate ratio |
|---|---|---|---|---|
|
Baseline |
37.3 |
38.1 |
- |
- |
|
Month 12 |
24.4 |
18.2 |
0.0244 |
6.646 (0.86 to 12.43) |
|
Month 24 |
14.3 |
25.1 |
0.0003 |
–10.745 (–16.50 to –4.98) |
|
Month 36 |
11.3 |
40.5 |
< 0.0001 |
–18.722 (–24.49 to –12.96) |
|
Month 48 |
9.2 |
44.2 |
< 0.0001 |
–24.582 (–30.35 to –18.82) |
|
Month 60 |
8.5 |
44.4 |
< 0.0001 |
–23.959 (–29.72 to –18.20) |
Molecular response according to European LeukemiaNet (ELN) criteria
Molecular response rates (Table 4) were significantly higher in the ropeginterferon alfa-2b arm in comparison with the control arm starting from Month 24 up to Month 60.
Table 4. Molecular response (using LOCF) according to ELN criteria1
|
CI, confidence interval; ELN, European LeukemiaNet; LOCF, last observation carried forward. |
||||
|
Study month |
Ropeginterferon alfa-2b (n = 95) |
Control (n = 76) |
p value |
Rate ratio |
|---|---|---|---|---|
|
Month 12 |
41/94 (43.6) |
36/73 (49.3) |
0.3744 |
0.87 (0.63─1.19) |
|
Month 24 |
64/94 (68.1) |
24/74 (32.4) |
0.0001 |
2.00 (1.41─2.84) |
|
Month 36 |
62/94 (66.0) |
20/74 (27.0) |
< 0.0001 |
2.31 (1.56─3.43) |
|
Month 48 |
63/94 (67.0) |
19/74 (25.7) |
< 0.0001 |
2.50 (1.67─3.73) |
|
Month 60 |
65/94 (69.1) |
16/74 (21.6) |
< 0.0001 |
3.04 (1.96─4.71) |
Between Month 24 and Month 60, analysis of the combined endpoint of achieving HCT < 45% without phlebotomy and molecular response always showed significantly higher response rates in the ropeginterferon alfa-2b arm vs the control arm:
- Month 24 (LOCF), 55.3% vs 24.3% (rate ratio [RR], 2.26; 95% CI, 1.46─3.50; p = 0.0003)
- Month 36 (LOCF), 57.5% vs 25.7% (RR, 2.17; 95% CI, 1.43─3.29; p = 0.0003)
- Month 48 (LOCF), 57.5% vs 20.3% (RR, 2.79; 95% CI, 1.74─4.46; p < 0.0001)
- Month 60 (LOCF), 58.5% vs 17.3% (RR, 3.26; 95% CI, 1.97─5.42; p < 0.0001)
Safety
For safety, the data lock date was May 29, 2020 (up to 6.3 years of treatment overall).
- The incidence of thromboembolic adverse events (AEs) over the entire treatment period was 1.2% per patient year in both arms
- The incidence of disease progression was 0.2% per patient year in the ropeginterferon alfa-2b arm (myelofibrosis, n = 1) vs 1.0% per patient year in the control arm (total, n = 4; myelofibrosis, n = 2; acute leukemia, n = 2)
An overview of the safety profile is shown in Figure 1.
Figure 1. Safety profile1
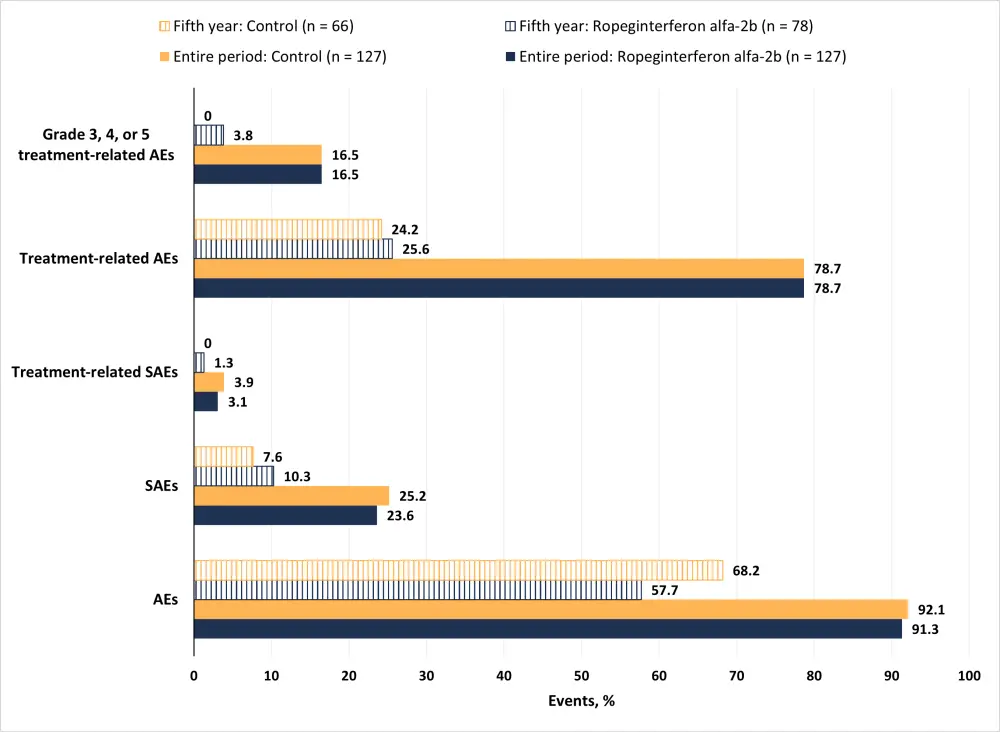
AE, adverse event; SAE, serious AE.
Treatment related AEs of special interest to interferon therapy in the ropeginterferon alfa-2b arm (n = 127) were seen for: endocrine organs (4.7%), musculoskeletal and connective tissue (1.6%), skin and subcutaneous tissue (1.6%), psychiatric disorders (0.8%), and immune system and blood and lymphatic system (0.8%).
Conclusion
- This long-term analysis showed that ropeginterferon alfa-2b is effective in patients with PV, with deep and durable responses
- Molecular responses continued to improve overtime up to Month 60, while responses in the control arm started to deteriorate at Month 24
- Patients in the ropeginterferon alfa-2b arm were more likely to be phlebotomy-free
- Only one patient progressed during long-term ropeginterferon alfa-2b therapy
- Ropeginterferon alfa-2b was well tolerated over long-time treatment, showing similar rates of treatment-related AEs compared to the control arm
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

