All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Ropeginterferon alfa-2b is effective and safe in patients older than 60 years with PV
As discussed in our last editorial theme, the pegylated forms of interferon-alpha improve treatment compliance and lower the needed dosing. This leads to a better safety profile, especially for older patients with myeloproliferative neoplasms (MPN).
In January 2020, Heinz Gisslinger and colleagues reported the results of the phase III trial, PROUD-PV, and its extension study, the CONTINUATION-PV trial.1 Please refer to this previous article on the MPN Hub for a summary of the study design, patient baseline characteristics, and key clinical outcomes. Here, we summarize the recently published subanalysis of the PROUD-PV trial on the use of ropeginterferon alfa-2b (ropeg) versus hydroxyurea (HU) in patients with polycythemia vera (PV) according to their age.2
Subanalysis of ropeginterferon alfa-2b according to age2
Age subanalysis included all patients enrolled in the CONTINUATION-PV study:
- A total of 254 patients were randomized 1:1 to receive ropeg or HU for 1 year in the PROUD-PV trial (NCT01949805). Most were included subsequently in the CONTINUATION-PV extension study (NCT02218047; N = 171) and continued their treatment with ropeg or best available therapy (98.4% of the control arm continued with HU) as long as it was safe and effective (study ongoing).
Overall, the study population was considered young, with a median age of 58 years and 59 years in the ropeg and HU arms, respectively. When grouped into < 60 years and ≥ 60 years, age groups were balanced for the most relevant patient characteristics.
After 24 months from ropeg treatment initiation, patients ≥ 60 years received a median dose of 500 μg every 2–4 weeks, while patients < 60 received a median of 350 μg. In the control arm, both groups received HU at a median dose of 1000 mg/day.
Efficacy
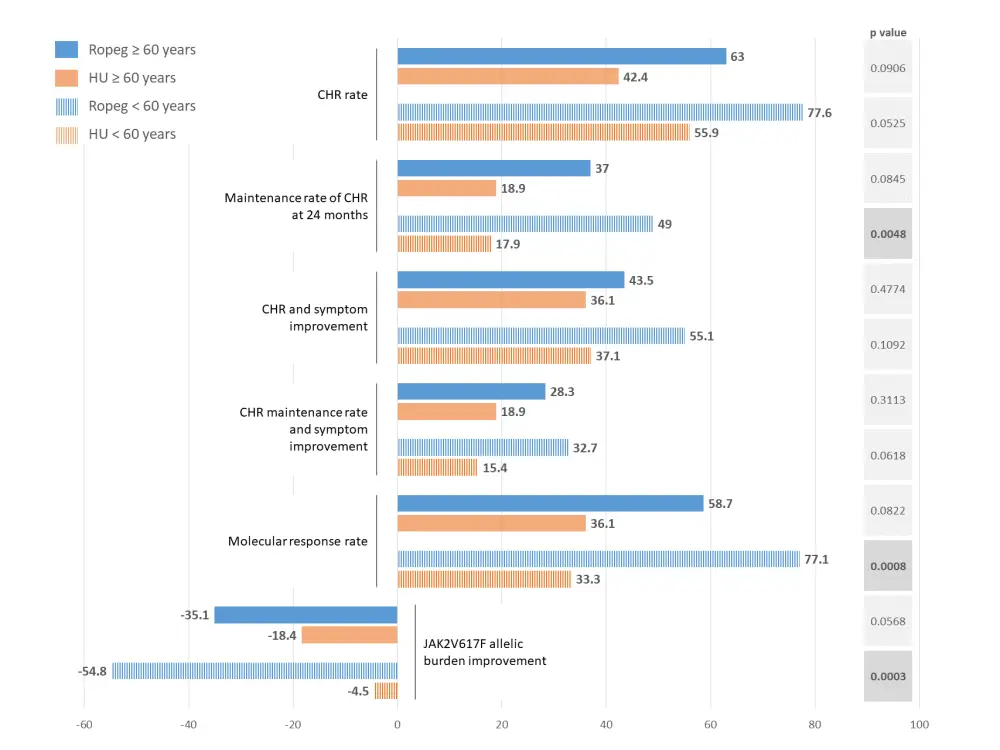
- Ropeg achieved significantly higher rates of complete hematological responses (CHR), maintenance of CHR at 24 months, and molecular responses regardless of age. CHR was defined as reported previously by Quintás-Cardama et al.3
- Patients ≥ 60 years showed a positive trend toward disease-related symptom improvement and reduced JAK2V617F allele burden, but differences with the control arm were smaller than those seen in the younger subgroup (Figure 1).
- Patients older than 70 years benefited from ropeg treatment similarly to the ≥ 60 years subgroup; however, due to the small sample size interpretation of data is difficult (nine patients treated with ropeg and 12 with HU).
Safety
- The adverse event (AE) incidence of any grade was comparable between treatment arms and age subgroups. However, patients ≥ 60 years experienced more serious AEs than younger patients, leading to a higher AE-related discontinuation rate (in the ropeg arm, ≥ 60 years: 6.5%; < 60 years: 0%).
- By contrast, when analyzing AEs associated with treatment, patients ≥ 60 years treated with ropeg experienced fewer AEs (63%) compared to those treated with HU (89.2%) and even compared to younger patients (ropeg, 77.6%; HU, 74.4%)(Figure 1).
Figure 1. Efficacy and safety of ropeg vs HU in patients with PV2
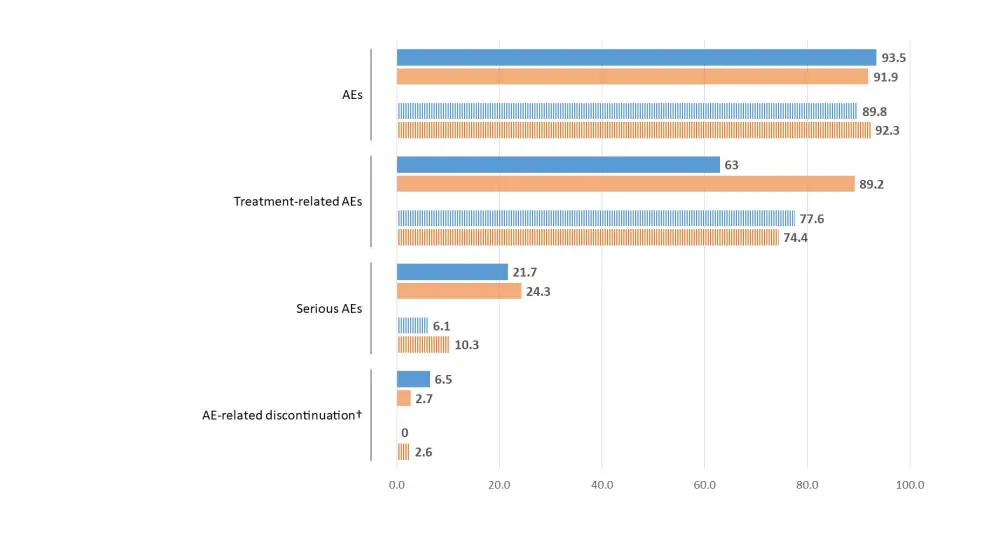
†Including one death.
Conclusion
The PROUD-PV and CONTINUATION-PV phase III trials led to the approval of ropeg in Europe as monotherapy for PV without symptomatic splenomegaly, and it is currently under evaluation by the U.S. Food and Drug Administration (FDA). A final decision is expected to take place soon.
This subanalysis on the efficacy and safety according to age confirms that ropeg is safe and efficacious in patients older than 60 years. Due to its favorable safety profile and good compliance, the authors advocate that ropeg should substitute HU in the current treatment recommendations for high-risk PV.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

