All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Clinical factors associated with response to ruxolitinib in patients with myelofibrosis
A dysregulated Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway is characteristic of myelofibrosis (MF). Ruxolitinib, a potent JAK1/JAK2 inhibitor, was approved in 2011 for the treatment of primary and secondary intermediate-/high-risk MF. The approval was based on the results of the pivotal phase III COMFORT studies in patients with intermediate-2- or high-risk MF. In these studies, ruxolitinib demonstrated superiority over placebo and best available therapy with durable improvements in splenomegaly, MF symptoms, and quality of life (QoL), and was also associated with better overall survival.
Due to limited data on patients with intermediate-1-risk MF, the phase IIIb expanded-access JUMP study (NCT01493414) was initiated with the aim to collect safety and efficacy data for ruxolitinib in this population of patients. This study enrolled 2,233 patients, and initial results from patients treated with ruxolitinib for ≥ 1 year (n = 1,144), including those with intermediate-1-risk disease, showed reductions in spleen length and MF symptoms.1
A recent publication in Leukemia & Lymphoma by Vikas Gupta further evaluated clinical factors associated with response to ruxolitinib using data from the JUMP study.2 Here, we report key findings from this study.
Study design1,2
The JUMP study is an open-label, multicenter, single-arm, phase IIIb expanded-access study in patients with MF. Eligibility criteria and study design are shown in Figure 1.
The starting doses of ruxolitinib were based on platelet count at baseline (Figure 1).
Spleen response was assessed using the International Working Group–Myeloproliferative Neoplasms Research and Treatment (IWG–MRT) criteria.
Endpoints
The primary endpoint was safety and tolerability of ruxolitinib.
Additional endpoints included:
- Proportion of patients with a ≥ 50% reduction in palpable spleen length
- Change from baseline in patient-reported symptom response:
- Functional Assessment of Chronic Illness Therapy–Fatigue [FACIT-F] scale
- Functional Assessment of Cancer Therapy–Lymphoma [FACT-Lym] total score
Figure 1. Study design1,2
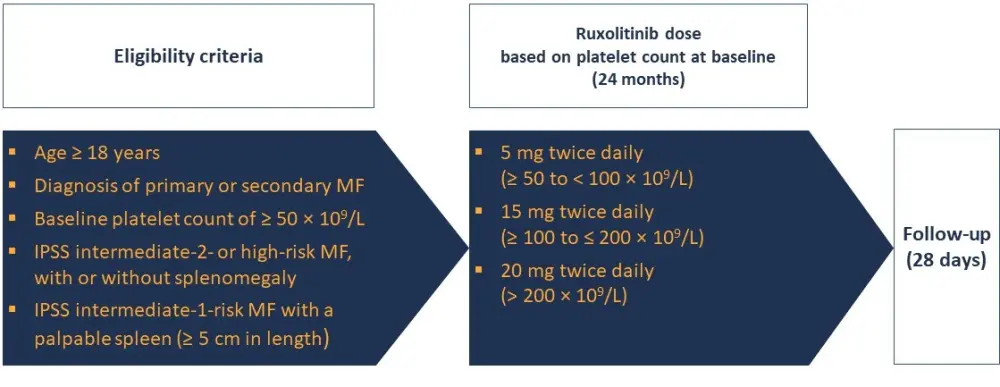
IPSS, International Prognostic Scoring System; MF, myelofibrosis.
Patient characteristics2
Baseline characteristics of the 2,233 patients treated in the JUMP study from 2011 to 2017 and included in this analysis are shown in Table 1.
- Mean FACT-Lym total score at baseline, 113.9; mean FACIT-F score, 32.7
Table 1. Baseline characteristics of patients2
|
DIPSS, Dynamic International Prognostic Scoring System; Hb, hemoglobin; IPSS, International Prognostic Scoring System; MF, myelofibrosis; SD, standard deviation; WBC, white blood cell. |
|
|
Characteristic |
Patients (N = 2,233) |
|---|---|
|
Median age (range), years |
67.0 (18–89) |
|
Mean time since initial diagnosis (SD), months |
51.7 (64.4) |
|
MF subtype, n (%) |
|
|
IPSS risk, n (%) |
|
|
DIPSS risk, n (%) |
|
|
Hb level, mean (SD), g/dL |
10.9 (2.3) |
|
Platelet count, mean (SD), × 109/L |
318.9 (238.6) |
|
Palpable spleen length, median (range), cm |
12.0 (0.5–45.0) |
Results2
Data on drug exposure are shown in Table 2.
Table 2. Ruxolitinib exposure2
|
Ruxolitinib exposure |
Patients |
n evaluable |
|---|---|---|
|
Median duration of exposure, months (range) |
12.4 (< 0.1─59.7) |
2,233 |
|
Mean daily dose, mg |
28.7 |
2,233 |
|
Starting dose, % |
|
2,233 |
|
Ruxolitinib dose > 20 mg/day at Week 12, % |
|
|
|
Exposure to ruxolitinib, % |
|
2,101 |
Spleen response
- A reduction in spleen size was observed in most patients, with a mean change from baseline of –68.3% (median, –75.0%; range, –100.0% to 133.3%) in the 2,049 patients assessed
- Factors predicting higher spleen response rates in univariate analysis were generally associated with:
- Better disease status (International Prognostic Scoring System [IPSS] intermediate-1/low risk status, Hb level ≥ 10 g/dL, platelet count ≥ 100 × 109/L, blasts < 1%)
- Early treatment initiation with ruxolitinib (time since MF diagnosis of ≤ 2 years), ruxolitinib as first-line treatment, ruxolitinib dose > 20 mg/day
- Prior treatment vs no prior treatment, only in patients in the Dynamic IPSS (DIPSS) intermediate-2/high-risk group (37.3% vs 26.8%; adjusted odds ratio [aOR], 0.62; 95% CI, 0.45–0.84)
- Of these, only three factors were confirmed in a multivariate analysis to predict higher spleen response rates (Table 3):
- IPSS intermediate-1/low
- Ruxolitinib as first-line treatment
- Ruxolitinib dose of > 20 mg/day at Week 12
Table 3. Factors associated with higher spleen response rates in multivariate analysis2
|
CI, confidence interval; IPSS, International Prognostic Scoring System; OR, odds ratio. |
|||
|
Factor predicting higher spleen response rates |
n/N |
% |
OR (95% CI) |
|---|---|---|---|
|
IPSS |
|
|
|
|
Prior treatment |
|
|
|
|
Ruxolitinib dose at Week 12 |
|
|
|
Factors predicting symptom improvement
- In the univariate analysis, the only factor predicting symptom improvement was a titrated dose of ruxolitinib of > 20 mg/day at Week 12.
- FACT-Lym total score-based symptom response achieved, ≤ 20 mg/day vs > 20 mg/day: 48.2% vs 56.7% (OR, 0.71; 95% CI, 0.57–0.88)
- FACIT-F scale-based symptom response achieved, ≤ 20 mg/day vs > 20 mg/day: 44.9% vs 51.5% (OR, 0.77; 95% CI, 0.62–0.96)
- No correlation with ruxolitinib dose and symptom score improvement was seen in the multivariate analysis
Overall survival
- The median overall survival was not reached in patients in the DIPSS intermediate-1/low risk group
- In patients in the DIPSS intermediate-2/high risk group, overall survival was 229.1 weeks vs 253.6 weeks in those who achieved a spleen response at Week 24 vs those who did not respond. However, because of the small sample size (with only 32 patients achieving spleen response), these results should be interpreted with caution
Conclusion2
This analysis revealed that lower IPSS risk, doses of ruxolitinib > 20 mg/day, and ruxolitinib given as first-line therapy are independent factors associated with higher spleen response rates.
No correlation between dose or demographic factors and patient-reported symptom improvement under ruxolitinib treatment was seen.
The limitations of the study include the lack of MF-specific health-related quality of life data and of cytogenetic and molecular data, and the limited length of follow-up time.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

