All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Current treatment experiences of ropeginterferon alfa-2b for patients with MPN
Philadelphia-negative myeloproliferative neoplasms (MPN) are a group of heterogeneous disorders characterized by the overproliferation of myeloid lineage cells.1 The most common types of MPN are polycythemia vera (PV), essential thrombocythemia, and primary myelofibrosis (MF), with PV being the most prevalent. Interestingly, >95% of patients diagnosed with PV have the JAK2V617F mutation. This plays a key role in disease pathogenesis by disrupting the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway leading to uncontrolled hematopoietic stem cell proliferation.
Currently, patients who are diagnosed with PV have limited therapeutic options and treatment with interferon therapy remains the only approach to demonstrate induction of both hematologic and molecular remission. In fact, ropeginterferon alfa-2b (ropegIFN alfa-2b) is the only approved pegylated interferon treatment for patients diagnosed with PV without symptomatic splenomegaly in the Czech Republic.1
In response to this, Podstavková et al.1 performed a retrospective analysis investigating the latest data around the use of ropegIFN alfa-2b in regular clinical practice compared with outcomes observed in controlled clinical trials. We summarize their findings below.
Study design
This retrospective, non-interventional analysis evaluated patients observed at the Department of Internal Medicine – Hematology and Oncology in Brno between May 2020 and July 2022. Areas of investigation included:
- Therapeutic dosage
- Efficacy
- Safety
All patients were administered treatment every 14 days, with one patient progressing to maintenance therapy during the monitoring period, which entailed reduced treatment once every 4 weeks. The initial doses were as follows:
- 11 patients on 50 µg
- 3 patients on 100 µg
The doses were then increased according to efficacy and tolerance (median, 150 μg; min, 50 μg; max, 250 μg).
Results
A total of 14 patients were included in the analysis, with a median age of 51 years. Most patients had a PV diagnosis, followed by essential thrombocythemia and then secondary MF (Table 1).
Table 1. Patient characteristics*
|
ET, essential thrombocythemia; MF, secondary myelofibrosis; PV, polycythemia vera. |
|
|
Characteristic, n (unless otherwise stated) |
All patients |
|---|---|
|
Sex |
|
|
Male |
10 |
|
Female |
4 |
|
Median age, years |
51 |
|
Diagnosis |
|
|
PV |
11 |
|
ET |
2 |
|
Secondary MF |
1 |
|
Low thrombotic risk |
10 |
|
High thrombotic risk |
4 |
|
Cytoreductive therapy |
|
|
1 line |
0 |
|
2 lines |
3 |
|
≥3 lines |
11 |
|
Median baseline hematocrit value, % |
43.5 |
|
Median baseline leukocyte count, × 109/L |
6.8 |
|
Median baseline thrombocyte count, × 109/L |
391 |
The median time from diagnosis to treatment onset was 21 months, while the median duration of therapy was 266 days. No patient received ropegIFN as a first-line treatment. All patients experienced stabilization and gradual reduction of hematocrit values with ropegIFN alfa-2b treatment. The JAK2V617F allele burden also decreased over time during the monitored period (Figure 1) and 11 patients experienced a complete hematologic response, with the median time to this response being 5 months.
Figure 1. Median JAK2V617F allele burden*
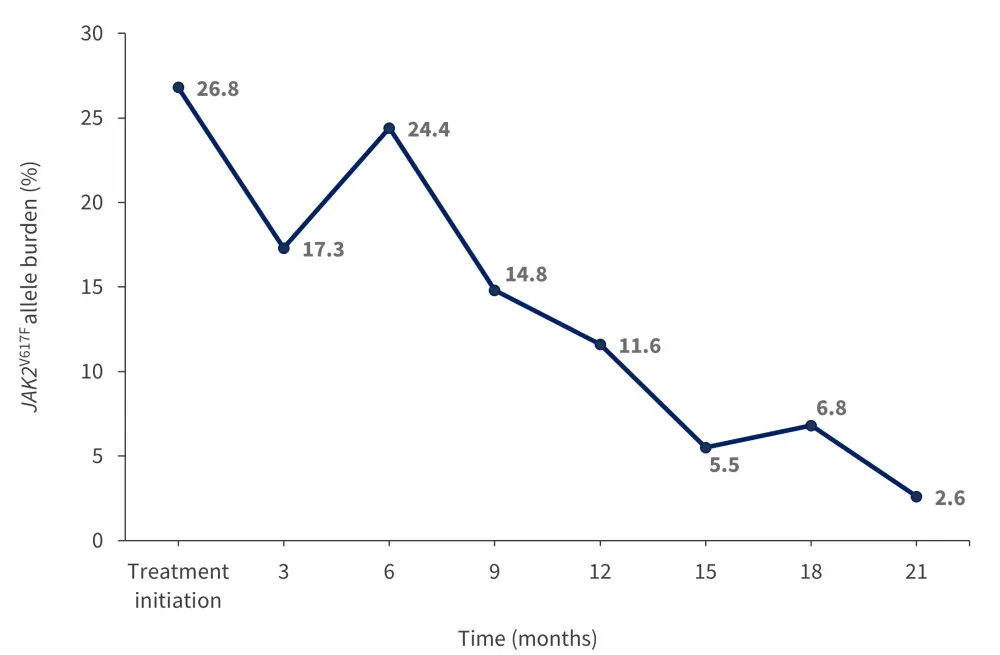
*Adapted from Podstavková, et al.1
Throughout the course of the monitoring period, treatment with ropegIFN was discontinued for two patients. The first was diagnosed with PV and had progressed to secondary MF and the other terminated treatment due to health insurance limitations. Adverse events (AEs) were recorded in six patients within the monitoring timeframe (Figure 2).
Figure 2. Adverse events observed in patients receiving ropeginterferon alfa-2b*
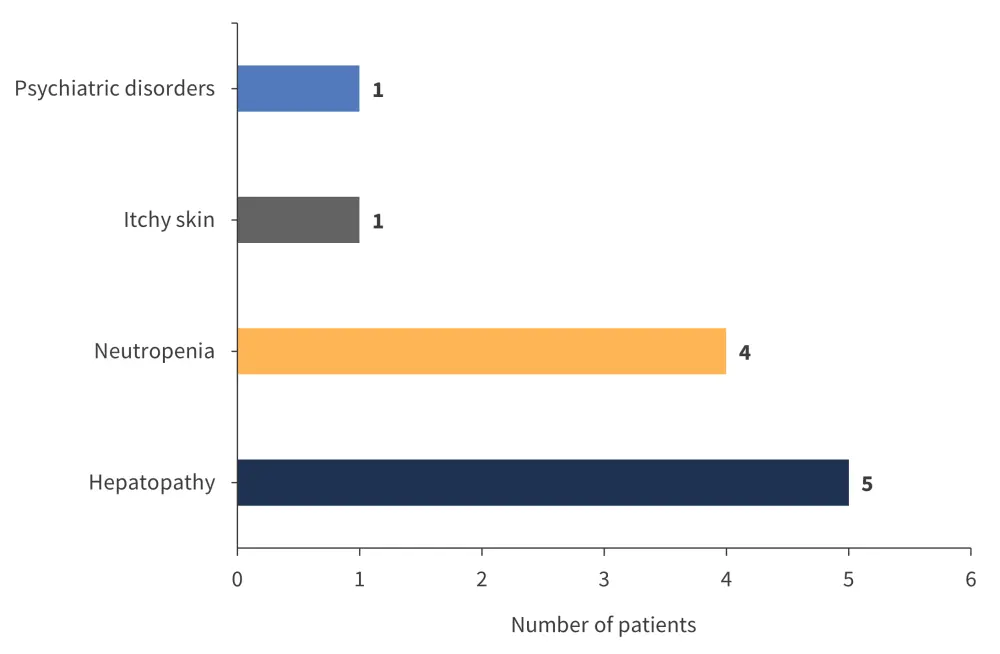
*Adapted from Podstavková, et al.1
All AEs observed in this analysis were of low grade according to the Common Terminology Criteria for AEs, with Grade 2 being the highest recorded. There were no flu-like symptoms in any patients and no discontinuation or termination of treatment due to AEs or intolerance. There was one thromboembolic complication that developed during a course of pneumonia brought on by the SARS-CoV-2 virus.
Conclusion
This analysis highlighted a reduction in allele burden and hematocrit value as well as disease stabilization in patients with MPN treated with ropegIFN alfa-2b. Lower doses of ropegIFN alfa-2b were also sufficient to achieve clinical responses in comparison with trial data. In addition to the low frequency of adverse effects, improved tolerability, and good patient compliance, ropegIFN alfa-2b treatment is considered efficacious and safe.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

