All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Defining disease modification with novel targeted agents in MF
Current targeted therapy options approved for the treatment of myelofibrosis (MF) by the U.S. Food and Drug Administration (FDA), namely the JAK1/2 inhibitor ruxolitinib, and the JAK2 inhibitor fedratinib, have demonstrated improvements in disease-related symptoms; however, relapse/refractoriness remains a hurdle. These factors encourage the better definition of clinical trial endpoints that incorporate disease modification in MF, rather than just symptom relief; nevertheless, there is no accepted assessment or consensus in defining disease modification in MF.
In a recent review article published by Naveen Pemmaraju et al.1 in Cancer, disease modification and associated response criteria in MF were defined. We summarize key points below.
Importance of JAK/STAT signalling
It is well understood that disruption of JAK/STAT signalling is a key driver of myeloproliferative neoplasms (MPN) pathology. When observing symptoms, initial activation of JAK/STAT signalling can increase myeloproliferation of megakaryocytes which aggregate in the bone marrow. This results in the infiltration of neutrophils, producing cytokine release and a pro-inflammatory microenvironment in the bone marrow. On the other hand, this promotes bone marrow fibrosis (BMF) and causes hematopoietic progenitor cells to migrate to extra medullary sites, thus driving splenomegaly. Despite the influence on disease course in the frontline setting, approved JAK inhibitors fail to disrupt disease progression, with clonal expansion and BMF remaining unaffected. As a result, trials investigating these compounds focus on total symptom score (TSS) and spleen volume reduction (SVR) ≥35/50% to infer efficacy.
Clearly, further options that may compliment these regimens and target other pathological mechanisms at an earlier stage are needed.
Allogeneic stem cell transplantation
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only potentially curative option for MF; however, a standardized view of clinical endpoints that enables patients to be identified as eligible for transplant remains unclear. To this end, measurable residual disease (MRD) is used for other hematologic malignancies, and molecular remission provides evidence of halting disease progression.
Clinical trials for novel targeted agents
The MPN Hub recently provided an overview of novel targeted agents for MF. This review provided a summary of clinical trials currently underway targeting different mechanisms that support MF progression. Such studies have started to add endpoints of BMF reduction, cytokine modulation, and mutational status, all of which provide evidence of disease modifying potential. Despite this, precise definition of these endpoints varies across clinical trials and the exact association with standard endpoints, such as overall survival (OS) and progression-free survival (PFS), need clarification.
New clinical trial endpoints
Disease modification is objectively difficult to measure; nevertheless, the authors of the review article1 proposed a definition of modification of MF stating “Disease modification in MF is defined as therapy that exerts a clinically meaningful impact on survival outcomes and/or restoration of normal haematopoiesis in conjunction with improvement in bone marrow fibrosis through a substantial and durable reduction in the clonal burden of disease.” Key modifiers are summarized in Figure 1, alongside a definition of disease modification suggested by the authors.1
Figure 1. Key disease modifier outcomes suggested for measuring in MF*
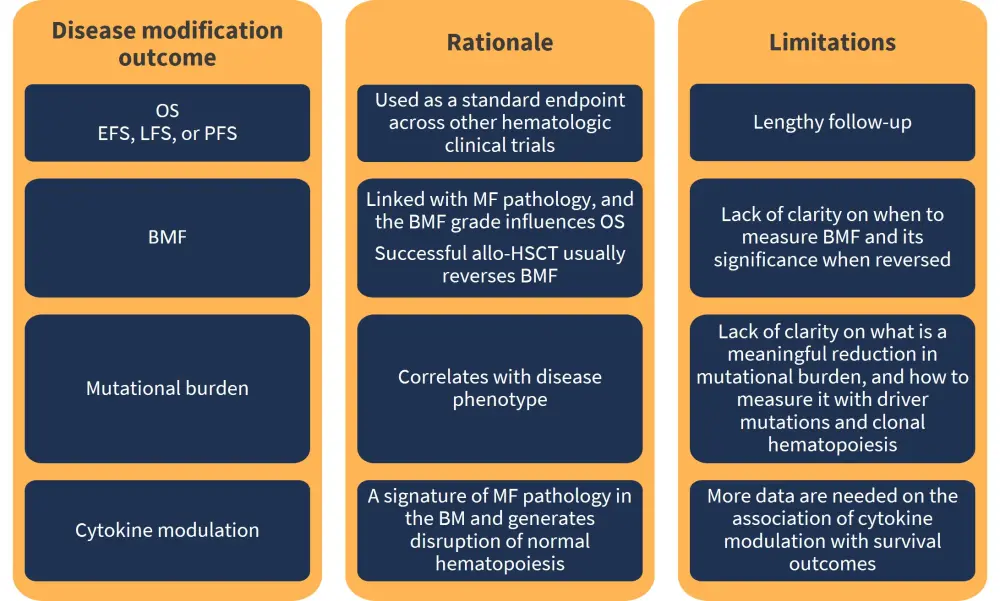
allo-HSCT, allogeneic hematopoietic stem cell transplantation; BM, bone marrow; BMF, bone marrow fibrosis; EFS, event-free survival; LFS, leukemia-free survival; MF, myelofibrosis; OS, overall survival; PFS, progression-free survival.
*Adapted from Pemmaraju et al.1
Conclusion
When considering new disease modifying endpoints in MF, the authors1 highlighted the following key points: Firstly, JAKi therapy will continue to remain a valid option despite its lack of impact on disease progression, and combinations of JAK inhibitors with novel agents is a likely treatment strategy to be investigated in clinical trials; Secondly, novel targeted agents have demonstrated evidence for disease modification, however a more unified approach to defining endpoints is needed across trials. Identifying such changes early in treatment may help to accelerate approval for promising agents.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

