All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Updates on non-JAK inhibitors for MF
Inhibiting the Janus kinase (JAK) pathway is considered the hallmark in treating myelofibrosis (MF); however, treatment discontinuation due to adverse events has been a concern. After JAK failure, therapeutic options are limited and there is an ongoing effort for therapies beyond JAK inhibitors (JAKi). Several non-JAK inhibitor therapies are in clinical development and have demonstrated acceptable safety and attenuation of splenomegaly and other key symptoms.
During the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, updates from clinical trials investigating non-JAKi were presented; and we provide a combined summary of these talks.
The MPN Hub have published an editorial theme on treatment options beyond JAK inhibition, which can be found here.
Bomedemstat1
Bomedemstat is a lysine-specific-demethylase (LSD-1) inhibitor that has been investigated as a monotherapy in a trial (NCT03136185), which included patients with relapsed/refractory (R/R) MF, and outcomes from 86 patients were previously summarized on the MPN Hub during the 26th Congress of the European Hematology Association (EHA 2021), including study design and patient characteristics. At the 63rd ASH Annual Meeting and Exposition, Harinder Gill presented updated results from 89 patients. The latest findings were similar to those reported during EHA 2021; good level of tolerability, improvements on symptoms and anemia, and the mutant allele frequency for driver and high molecular risk (HMR) mutations, such as ASXL1, were stable or decreased in 81% of patients.
Bomedemstat is also currently under investigation for essential thrombocythemia (ET) and polycythemia vera (PV). For findings from patients with ET, read our summary here.
Tagraxofusp2
Tagraxofusp is a novel CD123-targeted therapy investigated in a phase I/II trial (NCT02268253) for patients with chronic myelomonocytic leukemia or MF, with high CD123 expression who have poor prognosis. Study design, patient characteristics, and data from 32 patients in the MF cohort (dose escalation stage, n = 4; and Stage 2, n = 28) were previously summarized on our hub. Since last update, an additional seven patients were included in the analysis, as reported at the 63rd ASH Annual Meeting and Exposition.
- Grade ≥3 treatment-emergent adverse events (TEAEs) were similar to the previous report with slight differences in thrombocytopenia (15%), and capillary leak syndrome (8%).
- Of 39 patients, 36 patients discontinued therapy, with disease progression and adverse events being the most common causes (26% and 23%, respectively).
- Response rates were also similar:
- Among 24 patients with baseline splenomegaly who were evaluated for spleen volume reduction (SVR), 54% achieved any SVR, and 29% achieved a ≥50% SVR.
- There was a meaningful reduction in total symptom score (TSS) in 56% (22/39) of patients, and 36% (14/39) of patients achieved a reduction of ≥50%.
- The median OS was 26.6 months (95% CI, 12.9–51.1), and 49% of patients remained alive at the data cutoff.
Pelabresib3
Pelabresib is a potent bromodomain and extra-terminal (BET) protein inhibitor that is currently investigated in the phase II MANIFEST trial (NCT02158858) in patients with MF in three arms; Arm 1 evaluates pelabresib monotherapy in patients who were resistant/intolerant or ineligible for JAKi treatment, which was divided into transfusion dependent (TD) and transfusion independent (TI) cohorts.
This update, at the 63rd ASH Annual Meeting and Exposition, provided results from Arm 1 with 36 patients treated in the TD cohort and 50 in the TI cohort (vs 16 and 27 patients, respectively, reported in the EHA 2020 update)
- In the TD cohort, the TD to TI conversion rate was 16% with a median time to conversion of 32 weeks.
- In the TI cohort, the overall hemoglobin response was 38.3%.
- JAKi R/R: 34%
- JAKi ineligible: 66%
- JAKi intolerant: 41%
- Spleen response was evaluated in 64 patients; 11% achieved SVR ≥35% (SVR35) and 31% achieved SVR ≥25% (SVR25).
- Median and mean spleen volume changes were −24% and −17%, respectively.
- A ≥50% reduction in TSS (TSS50) was seen in 28%, with a median and mean TSS change of −40%.
- Bone marrow fibrosis (BMF) was improved by one grade in 16.7% of patients, and ≥2 grades in 6.7% of patients. There was no change in BMF in 43.3%, and 10.0% had BMF worsening.
- 71% of patients with BMF improvement also achieved hemoglobin response.
- The most strongly downregulated cytokines were 21 cytokines involved in MF pathogenesis and TNFR2 non-canonical NF-kB pathway, and interleukin (IL)-10, IL-4, IL-13, IL-18 signaling.
- 59% of patients reported at least one Grade ≥3 TEAE.
- Grade ≥3 hematologic TEAEs were thrombocytopenia (Grade 3 = 19%; Grade 4 = 4%), and anemia (Grade 3 = 15%; none Grade 4), whereas nonhematologic TEAEs included diarrhea (6%), respiratory tract infection (5%), asthenic conditions (2%), pruritus (2%), and constipation (1%).
- TEAEs led to pelabresib discontinuation in 19% of patients.
The phase III MANIFEST-2 trial investigating pelabresib + ruxolitinib versus placebo + ruxolitinib in JAKi-naïve patients with MF is currently ongoing (NCT04603495).
AVID2004
AVID200 is a novel, highly specific TGF-β 1/3 inhibitor investigated in a phase Ib trial for patients with MF (NCT03895112). AVID200 has been investigated as a potential therapy due to multiple molecular avenues by which upregulation of TGF-β 1/3 promotes BMF. Notable mechanisms include inhibition of normal megakaryocyte formation and, therefore, platelet production.
Study design
Eligibility criteria for this study included:
- Resistance/intolerance to ruxolitinib
- DIPSS intermediate-2/high-risk disease
- Grade 2 or 3 BMF
- Adequate organ function
AVID200 was administered intravenously at three dosing levels on Day 1 of a 21-day cycle, and the response was assessed at Cycle 7.
- In the dose escalation stage, three doses were tested and in the absence of dose limiting toxicities (DLTs), the two top doses were expanded in 12 patients (Figure 1)
- Patients with a clinical response were able to continue with AVID200 therapy following Cycle 7
Figure 1. Dose escalation*
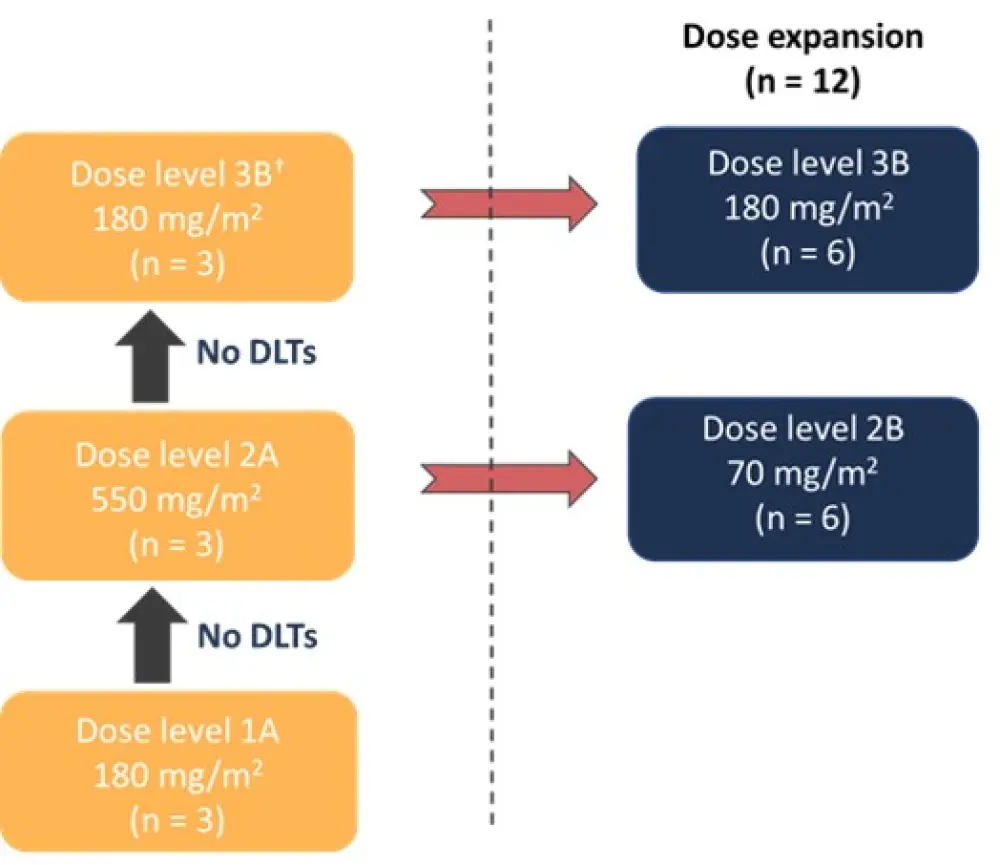
DLTs, dose limiting toxicities.
*Adapted from Mascarenhas et al.4
†Dose level 3A (1,100 mg/m2) was not used in the study due to drug supply shortage (was switched from A to B lot).
Baseline characteristics for all patients treated in the dose escalation (n = 9) and the dose expansion stage (n = 12) are summarized in Table 1.
Table 1. Patient characteristics*
|
CALR, calreticulin; DIPSS, Dynamic International Prognostic Scoring System; HMR, high molecular risk; TSS, total symptom score. |
|
|
Characteristic |
N = 21 |
|---|---|
|
Median age, years (range) |
73 (51–81) |
|
Prior ruxolitinib treatment, % |
90.0 |
|
DIPSS, % |
|
|
Intermediate-2 |
66.7 |
|
High-risk |
33.3 |
|
JAK2 V617F, % |
71.4 |
|
CALR, % |
19.0 |
|
TSS, median (range) |
14 (3–39) |
|
Patients with HMR mutation, % |
50 |
Efficacy
- At Cycle 7, two patients achieved clinical improvement (one achieving spleen, anemia, and symptom response, and one achieving TSS50), seven had stable disease, four had progression, and eight patients were not evaluable due to withdrawal prior to Cycle 7.
- Three out of four patients treated to Cycle 12 achieved clinical improvement with a reduction in TSS (n = 2), and SVR, anemia, and TSS (n = 1).
- Two patients remain on treatment.
- Four patients achieved a SVR50 from baseline, while nine patients reported TSS50.
- Three patients had an improvement in platelet count that was durable with treatment.
- Plasma TGF-β1 levels were lowered.
- Reductions in key inflammatory cytokines including IL-8, TNF-alpha, YKL-40, and p-selectin were variable.
- No reduction in BMF grade, cellularity, and megakaryocyte count was reported.
Safety
- AVID200 was well tolerated with no DLTs.
- 76.2% of patients had Grade 3/4 adverse events including thrombocytopenia (Grade 4, n = 1), anemia, fatigue, epistaxis, maculopapular rash, mucositis; eight of these events were thought to be potentially related to the study drug.
Selinexor5
Selinexor is a small, oral, selective inhibitor of nuclear export that blocks the karyopherin protein exportin1 (XPO1) that is currently being evaluated in the phase II ESSENTIAL study (NCT03627403) for patients with JAKi R/R MF.
Study design
- Patients with primary or secondary MF, who were resistant after at least 3 months on JAK inhibition, or intolerant to JAKi, were given low-dose oral selinexor (60–80 mg) once a week until disease progression.
- Study endpoints after 24 weeks were SVR35 (primary) and TSS50 (key secondary).
Results
12 patients were eligible for the analysis (Table 2).
Table 2. Patient characteristics*
|
CALR, calreticulin; HMR, high molecular risk; JAKi, Janus kinase inhibitor; MPL, myeloproliferative leukemia virus. |
|
|
Characteristic |
N = 12 |
|---|---|
|
Median age, years (range) |
68 (43–80) |
|
Median duration of JAKi therapy, months (range) |
22 (0.5–96) |
|
Resistance to JAKi, % |
92 |
|
Median spleen volume, cm3 (range) |
1,454 (835–5,792) |
|
Driver mutations, % |
|
|
JAK2 |
58.3 |
|
CALR |
33.3 |
|
MPL |
8.3 |
|
≥1 HMR mutation, % |
67 |
Efficacy
Median treatment duration was 11 months (2.8 – 28.8).
- Spleen responses in 10 evaluable patients were as follows:
-
- At week 24: SVR35 = 30%; SVR25 = 50%
- Beyond Week 24: SVR35 = 40%; SVR25 = 60%
- An improvement in hemoglobin levels was observed in four out of eight patients who had a baseline hemoglobin of <10 g/dL at screening.
- Of five TD patients at baseline, two (40%) became TI.
- A reduction in TSS was observed in eight evaluable patients; however, this analysis was difficult to perform due to virtual check-ups around the COVID-19 pandemic.
- Median OS was not reached after a median follow-up of 11.1 months (range, 3–30), and the 2-year survival probability was 91.7%.
- A decrease in white blood cell counts and lactose dehydrogenase levels was observed early on treatment indicating a reduction in myeloproliferative activity.
- There was no change in marrow reticulin or collagen fibrosis (except one patient who achieved a reduction from BMF Grade 3 to Grade 1) and JAK2 V617F allele burden at Week 24.
- Inflammatory cytokines were decreased in one patient who had high levels of cytokines at baseline.
- Eight patients (70%) discontinued treatment due to death, progression (enlarged spleen size and acute myeloid leukemia), enrollment to another trial, and toxicity (fatigue and weight loss).
Safety
Grade ≥3 TEAEs are summarized in Table 3. 10 patients had a dose reduction due to fatigue, anemia, thrombocytopenia, abdominal pain and, most commonly, weight loss.
Table 3. Grade ≥3 TEAEs*
|
TEAEs, treatment-emergent adverse events. |
|
|
Grade ≥3 TEAEs, % |
N = 12 |
|---|---|
|
Hematologic |
|
|
Anemia |
33 |
|
Thrombocytopenia |
17 |
|
Nonhematologic |
|
|
Weight loss |
8 |
|
Fatigue |
33 |
|
Dyspnea and hypoxia |
17 |
|
Hypertension |
17 |
|
Dizziness |
17 |
|
Flu-like symptoms |
17 |
|
Sepsis |
8 |
In summary, selinexor produced clinical responses in patients with JAKi-refractory MF. Long term use of selinexor appeared to be safe with manageable side effects. Further two studies will investigate selinexor in combination with ruxolitinib in patients with JAKi naïve MF (NCT04562389) and as a monotherapy in patients with previously treated MF (NCT04562870).
Sotatercept6
Sotatercept is a first-in-class, fusion protein composed of the extracellular domain of the human activin receptor and the Fc region of human IgG1, and traps ligands of the TGF-β superfamily that inhibit terminal erythropoiesis. Final results from a phase II trial investigating the safety and efficacy of sotatercept in anemic patients with MF (NCT01712308), were presented at the 63rd ASH Annual Meeting and Exposition.
Study design
Patients recruited in this study had primary or secondary MF and anemia, and sotatercept was given either as a monotherapy or in combination with ruxolitinib (Figure 2 and Table 4).
Figure 2. Study cohorts*
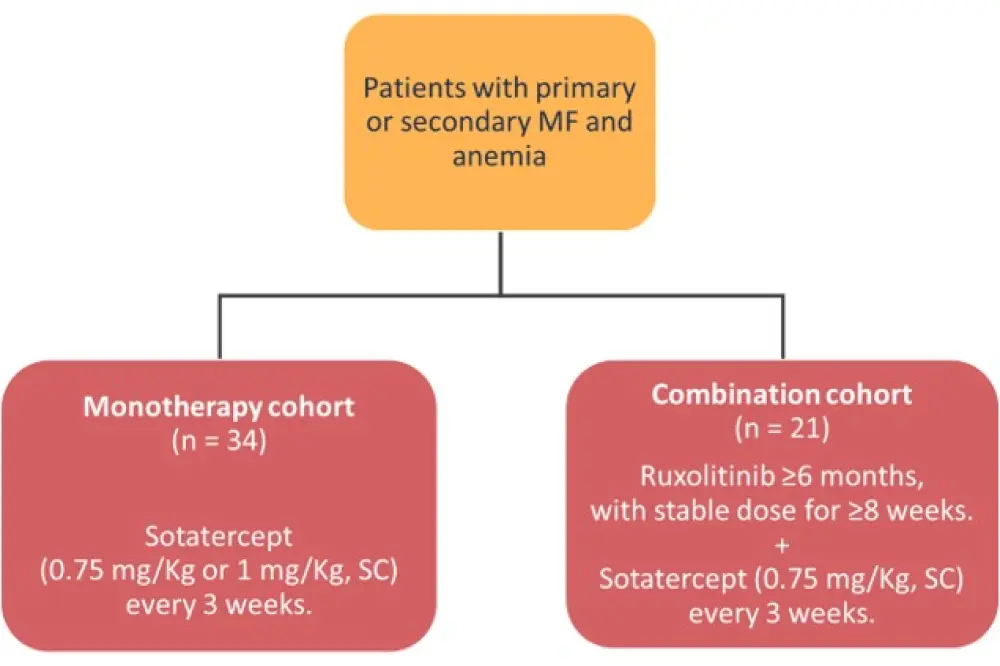
MF, myelofibrosis; SC, subcutaneously.
*Adapted from Bose et al.6
- Those on study treatment for ≥84 days or ≥12 weeks were evaluated.
- Primary endpoint was the red blood cell TI for patients who were TD at baseline, and hemoglobin improvement by an increase of 1.5 g/dL for ≥12 weeks for those who were TI at baseline.
Table 4. Patient characteristics by treatment cohort*
|
CALR, calreticulin; DIPSS, Dynamic International Prognostic Scoring System; Hgb, hemoglobin; MPL, myeloproliferative leukemia virus. |
||
|
Characteristic |
Sotatercept alone |
Sotatercept + ruxolitinib |
|---|---|---|
|
Median age, years (range) |
67 (47–84) |
71 (48–84) |
|
Median baseline Hgb, g/dL (range) |
7.4 (4.7–9.3) |
7.4 (4.6–9.1) |
|
Mutation, n |
||
|
JAK2 |
22 |
15 |
|
CALR |
4 |
4 |
|
MPL |
6 |
2 |
|
Triple-negative |
1 |
0 |
|
DIPSS, n |
||
|
Intermediate-1 |
2 |
0 |
|
Intermediate-2 |
27 |
20 |
|
High-risk |
5 |
1 |
|
Splenomegaly present, n |
19 |
12 |
|
Previously treated, n |
28 |
21 |
Efficacy
The overall response rate (ORR) for the monotherapy cohort (n = 27, evaluable patients) was 30% with a median duration of response (DoR) of 23.3 months (range, 3.9–68.4), whereas the ORR for the combination cohort (n = 19, evaluable patients) was 32% with a median DoR of 19.9 months (range, 3.7–56.8). Type of response was anemia and TI in the monotherapy cohort, and anemia only in the combination cohort. Four patients in the monotherapy cohort and three in the combination cohort required multiple dose holds for hemoglobin levels ≥11.5 g/dL, and sotatercept resumed when hemoglobin was <11 g/dL.
Safety
Sotatercept was well tolerated, with a total of nine Grade ≥3 events possibly related to treatment, including pain/myalgia (n = 2) and hypertension (n = 7). All but two patients (in each cohort) discontinued treatment, with the most common reason being lack or loss of response in both the monotherapy (n = 14) and combination (n = 8) cohort.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

