All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Discovery of a new anti-CALR mutant monoclonal antibody for the treatment of MPN
Mutations in the calreticulin (CALR) gene are responsible for 20–30% of myeloproliferative neoplasms diagnosed today.1 While this gene is not as prolific as the JAK2V617F genetic aberration, mutant CALR proteins are a strong driver of oncogenic cell proliferation resulting from the expression of a positively charged C‑terminus together with the loss of the KDEL endoplasmic reticulum retention signal.
The resulting mutant protein is able to form a stable complex with the thrombopoietin receptor (TPO-R), which is trafficked to the cell surface, leading to Janus kinase 2 (JAK2)/signal transducer and activator of transcription (STAT) signaling pathway activation.1
During the 64th American Society of Hematology Annual Meeting and Exposition, Reis gave a presentation on the discovery and development of a new anti-CALR mutant monoclonal antibody, INCA033989.2 We are pleased to summarize the presentation below.
New monoclonal antibody
INCA033989 is a fully human G1 immunoglobulin with a silent constant fragment domain. It has been engineered to selectively bind to mutant CALR with high affinity while not binding to wild type (WT) CALR. Once bound, the monoclonal antibody inhibits CALR mutant-dependent TPO-R dimerization, resulting in suppression of TPO-R signaling transduction and subsequent oncogenic cell proliferation through cell cycle arrest and cell death (Figure 1).
Figure 1. INCA033989 mechanism of action*
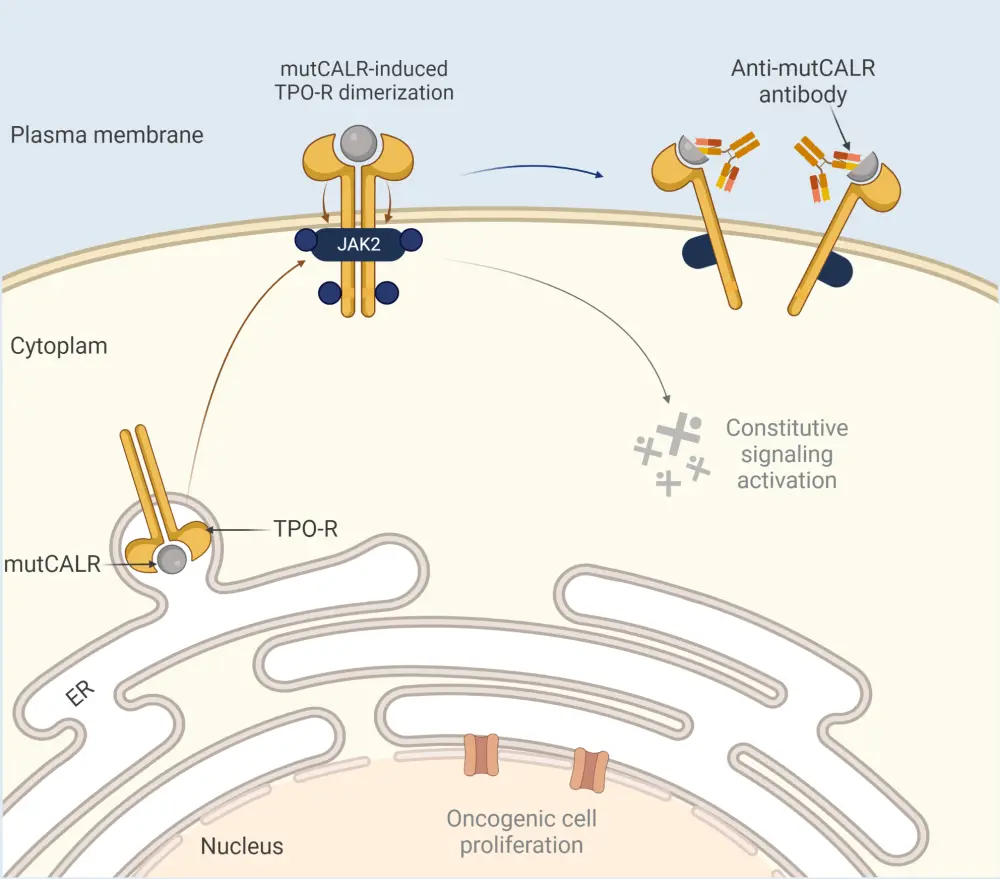
ER, endoplasmic reticulum; JAK2, Janus kinase 2; mutCALR, mutant calreticulin; TPO-R, thrombopoietin receptor.
*Adapted from Reis.2 Created with BioRender.com.
CD34+ cells
In the study presented by Reis, the effects of INCA033989 on CD34+ cells isolated from patients diagnosed with myeloproliferative neoplasms were investigated. INCA033989 was found to selectively inhibit phosphorylated-STAT5 in a dose-dependent manner in cells with mutant CALR while having no effect on cells harboring WT CALR or the JAK2V617F mutation.
Using the same CD34+ cells, the effects on cell differentiation and proliferation were also measured. Treatment with INCA033989 at increasing concentrations resulted in a significant decrease in the number of hematopoietic stem cells (HSP with mutant CALR, whilst having no effect on the proliferation of WT cells.
Within the megakaryocyte population (the lineage most effected by CALR mutations), cells with mutant CALR experienced a significant reduction in the rate of proliferation. In contrast, INCA033989 treatment had no effect on the proliferation of megakaryocytes harboring the JAK2V617F mutation. This confirms that INCA033989 acts on the disease-initiating clones, while selectively inhibiting the proliferation and differentiation of HSCs with mutant CALR.
Murine model
During investigation using essential thrombocythemia-induced murine models, INCA033989 was found to significantly reduce both total platelet and mutant CALR platelet expansion over 10 weeks compared with the isotype control, which demonstrated increased platelet and mutant CALR platelet expansion.
After 10 weeks of treatment, analysis of the bone marrow of mice treated with INCA033989 showed no sign of abnormal megakaryocytes, while mice treated with the isotype exhibited megakaryocyte hyperplasia characteristic of essential thrombocytopenia. Furthermore, the percentage of mutant HSCs was significantly reduced in mice treated with INCA033989 compared with those treated with the isotype. Despite significant inhibition of mutant CALR HSP, overall bone marrow cellularity was similar in both INCA033989-treated and isotype-treated mice, suggesting that WT HSCs were provided an opportunity for repopulation. There was also a lack of disease development in secondary transplant recipients who received bone marrow from mice treated with INCA033989, again suggesting the targeting of disease-initiating mutant CALR stem cells.
Conclusion
The new monoclonal antibody INCA033989 is a highly selective and potent antagonist of CALR mutant function, resulting in selective inhibition of JAK2/STAT signaling. The ability to target only disease initiating cells supports the rationale for clinical investigation. A phase I study is planned to begin in 2023.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

