All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Editorial theme | Meta-analysis of the effectiveness of anticoagulant therapy in patients with splanchnic vein thrombosis
Following on from our previous editorial theme article on the pathogenesis and management of splanchnic vein thrombosis (SVT) published on the MPN Hub here, we summarize an individual patient meta-analysis performed by Candeloro et al.1 evaluating the effectiveness of anticoagulant therapy in relation to recurrent venous thromboembolism and all-cause mortality, as well as safety relating to major bleeding in patients with SVT. Datasets from several prospective cohorts and random clinical trials obtained from Medline, Embase, and clinicaltrials.gov were merged and assessed.
Results
A total of 1,635 patients from three eligible studies were included in the final analysis. The complete set of patient characteristics is shown in Table 1. The mean age of the patients was 56 years, with 41.9% of patients diagnosed with SVT. The median follow-up time was recorded at 442 days and median treatment duration was 316 days. Patients treated with anticoagulant therapy had a lower frequency of several factors, including:
- solid cancers;
- liver cirrhosis; and
- symptomatic presentation of SVT.
Table 1. Patient characteristics*
|
DOAC, direct oral anticoagulant; LMWH, low molecular weight heparin; SD, standard deviation; UFH, unfractionated heparin; VKA, vitamin K antagonist. |
|
|
Variable, % (unless otherwise stated) |
All patients |
|---|---|
|
Mean age ± SD, years |
56.17 ± 16.16 |
|
Male |
60.3 |
|
Symptomatic patients |
58.1 |
|
Involved vein |
|
|
Portal vein |
34.4 |
|
Mesenteric vein |
13.3 |
|
Budd-Chiari syndrome |
12.3 |
|
Splenic vein |
6.5 |
|
Multiple veins |
33.5 |
|
Risk factors |
|
|
Unprovoked |
28.3 |
|
Solid cancer |
32.0 |
|
Liver cirrhosis |
17.6 |
|
Myeloproliferative neoplasm |
7.2 |
|
Recent surgery |
9.5 |
|
Thrombophilia |
|
|
Tested negative |
11.4 |
|
Tested positive |
17.2 |
|
Anticoagulant therapy |
|
|
None |
19.8 |
|
LMWH |
31.9 |
|
VKA |
25.4 |
|
DOAC |
1.7 |
|
UFH |
0.5 |
|
Fondaparinux |
1.0 |
|
Multiple agents |
19.6 |
Venous thromboembolism
At the time of follow-up, a total of 104 patients experienced a venous thromboembolism (VTE) at an incidence rate of 5.3 per 100 person-years. The cumulative incidence rates and types of VTE are shown in Figure 1 and Figure 2, respectively. The incidence rate of VTE was found to be lower during anticoagulant therapy compared with post-treatment discontinuation. The highest VTE incidence rate was recorded in patients who did not receive any anticoagulant therapy.
Figure 1. Cumulative incidence rates of VTE*
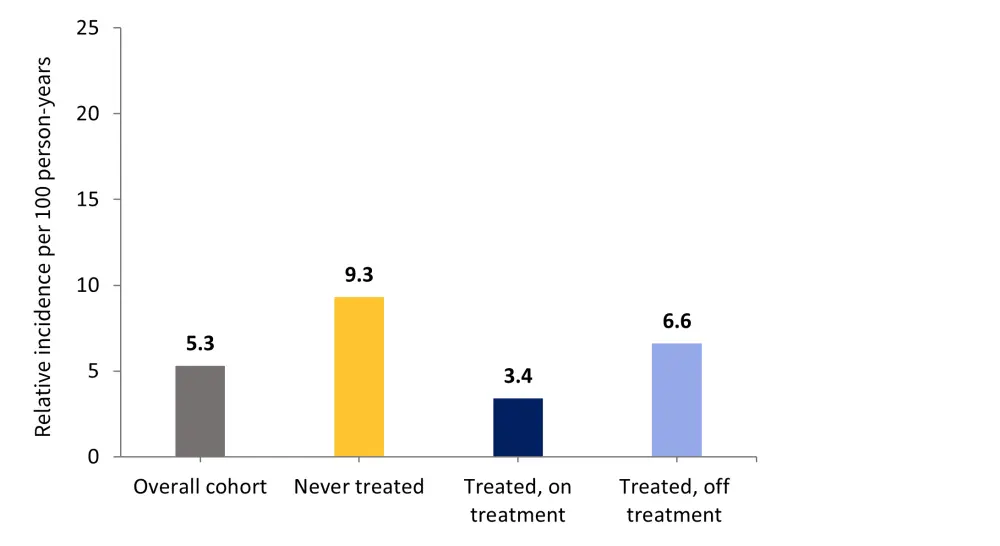
VTE, venous thromboembolism.
*Data from Candeloro, et al.1
Figure 2. Types of recurrent VTE*
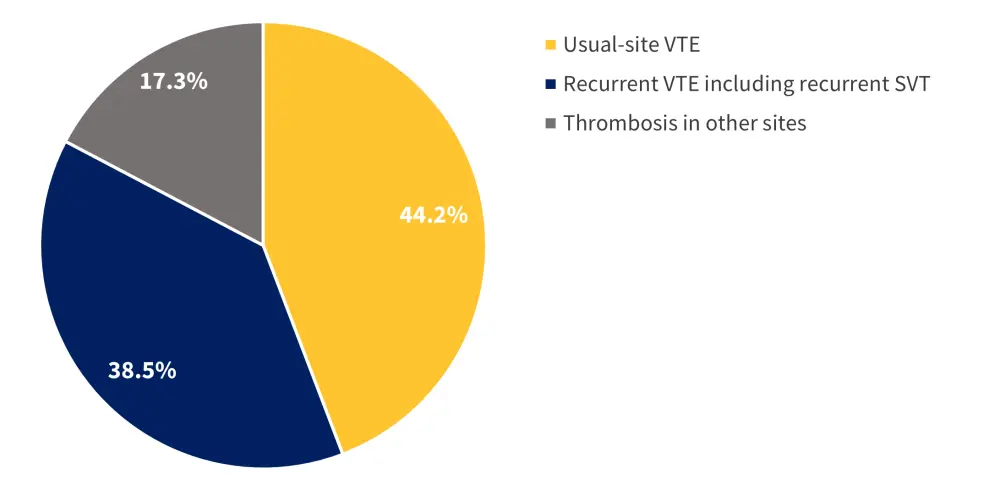
SVT, splanchnic vein thrombosis; VTE, venous thromboembolism.
*Data from Candeloro, et al.1
The number of patients who experienced recurrent VTE while receiving treatment is shown in Table 2.
Table 2. Number of recurrent VTE events associated with each treatment type*
|
DOAC, direct oral anticoagulants; LMWH, low molecular weight heparin; VKA, vitamin K antagonist. *Data from Candeloro, et al.1 |
|
|
Type of treatment |
Number of patients |
|---|---|
|
LMWH |
21 |
|
VKA |
16 |
|
Unfractionated heparin |
1 |
|
DOAC |
0 |
Major bleeding
Major bleeding occurred in 86 patients, and the median time to an event was 197 days, at an incidence rate of 4.4 per 100 person-years (Figure 3).
Figure 3. Cumulative incidence rates of major bleeding*
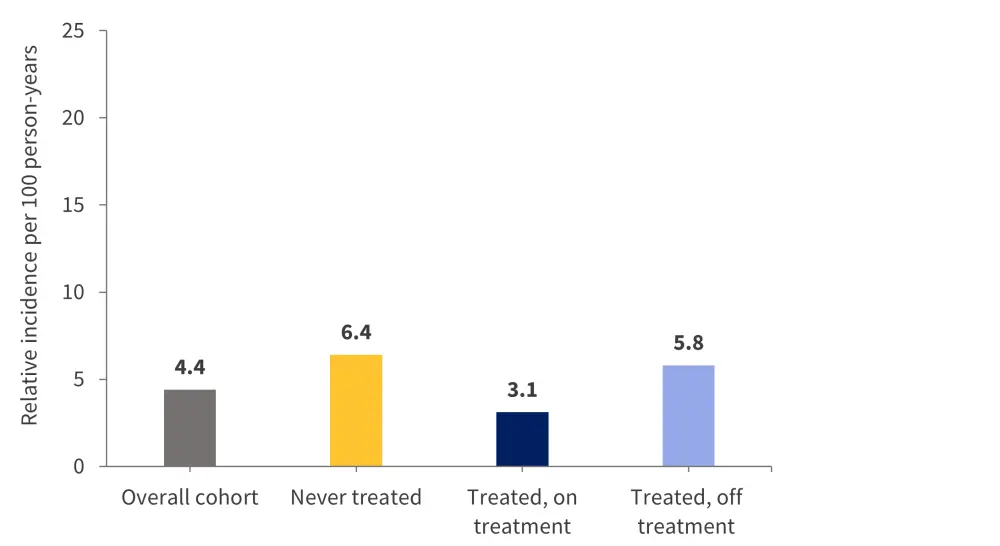
*Data from Candeloro, et al.1
A total of five fatal bleeding events were recorded (Table 3); four of these occurred in patients who were not treated with anticoagulant therapy and one occurred in a patient who withdrew from unfractionated heparin treatment.
Table 3. Causes of fatal bleeding events*
|
*Data from Candeloro, et al.1 |
|
|
Cause of bleeding |
Number of patients |
|---|---|
|
Gastroesophageal varices |
2 |
|
Nonvariceal gastrointestinal bleeding |
1 |
|
Unreported |
2 |
There were 23 bleeding events among patients treated with LMWH, 11 events among patients treated with VKA, and only one event among patients receiving DOAC.
All-cause mortality
A total of 256 patients died during the follow-up period, at an incidence rate of 13 deaths per 100 person-years. The causes of death are shown in Figure 4.
Figure 4. Causes of death*
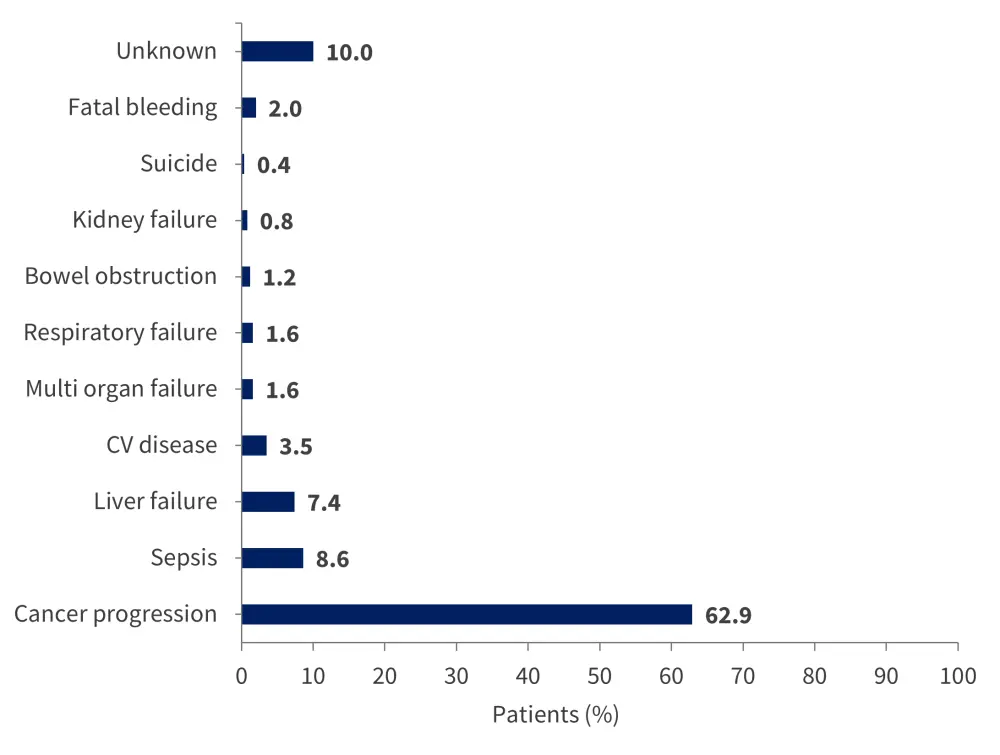
CV, cardiovascular.
*Adapted from Candeloro, et al.1
The incidence rate of deaths was lower for patients treated with anticoagulant therapy compared with patients who discontinued treatment, and highest in those who received no treatment (Figure 5).
Figure 5. Cumulative incidence rates of all-cause mortality*
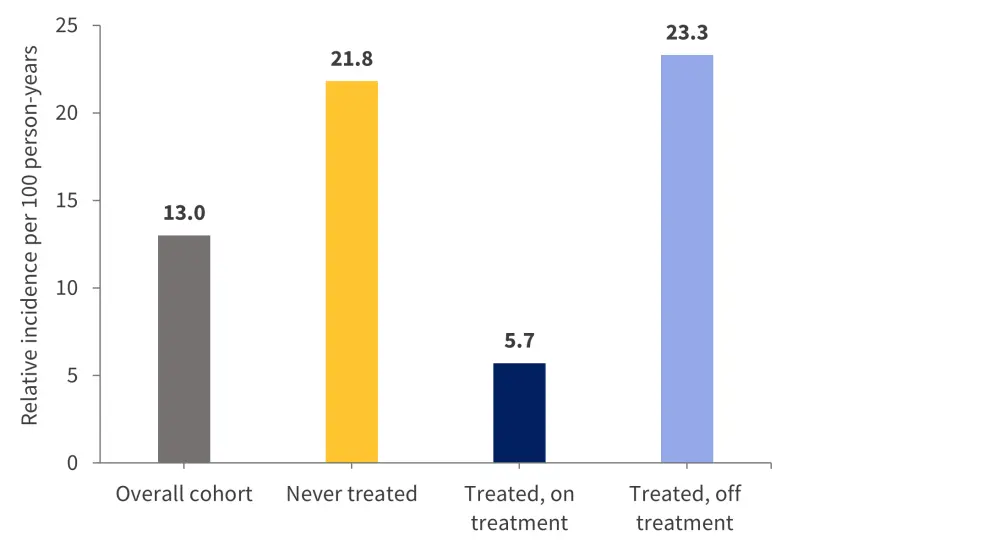
CI, confidence interval.
*Data from Candeloro, et al.1
Univariable and multivariable analysis
Analysis found that patients on anticoagulant therapy had a lower risk of:
- recurrent VTE;
- major bleeding; and
- all-cause mortality.
All factors were adjusted for sex, age, liver cirrhosis, solid cancer, MPN, and transient or persistent nonmalignant risk factors. However, the risk of recurrent VTE and all-cause mortality increased in patients with solid cancer, while the risk of major bleeding increased in patients with liver cirrhosis.
Conclusion
The crucial strengths of the study were the large population size, as well as time-varying and multivariable analyses, which allowed for a more precise estimate of the therapeutic effects. In contrast, there were several limitations that should be noted. The first is that some risk factors, such as prior VTE and bleeding, were poorly represented among the patient population and therefore the effects were not shown in the reported outcomes. Other variables including stage of thrombosis and prevalence of thrombophilia were also missing and were omitted from the final analysis. Furthermore, specific anticoagulant therapy regimens and decisions were not randomized or directed by protocol. Finally, the diversity in treatments offered to patients did not allow full analysis of effectiveness and safety in relation to specific treatments or doses. However, crucially, the collective results from the analysis highlight patients with SVT have a significant risk of recurrent VTE and major bleeding events. This risk appears to be lowered upon the administration of anticoagulant therapy.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

