All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Effect of allo-HSCT and BM fibrosis regression on OS in patients with MF
Patients diagnosed with intermediate-2 and high-risk myelofibrosis (MF) are currently only able to achieve curative therapy through allogeneic hematopoietic stem cell transplantation (allo-HSCT).1 Gandhi1 presented a study investigating the impact of pre-allo-HSCT splenomegaly, splenic irradiation, and pre- and post-HSCT marrow fibrosis on overall survival (OS) at the 2023 Transplantation and Cellular Therapy Meetings of ASTCT and CIBMTR, and we are pleased to summarize the key findings from the study below.
The MPN Hub recently published a related article summarizing the association between changes in bone marrow fibrosis and clinical efficacy of MF treatments here.
Study design
A total of 34 patients diagnosed with either primary or secondary MF and who underwent allo-HSCT between 2005 and 2021 were enrolled in the study. Baseline patient characteristics are shown in Table 1.
Table 1. Baseline patient characteristics*
|
allo-HSCT, allogeneic hematopoietic stem cell transplant; DIPSS, Dynamic International |
|
|
Characteristic, % (unless otherwise stated) |
N = 34 |
|---|---|
|
Age at allo-HSCT (range), years |
62 (42–73) |
|
KPS |
|
|
90 |
32.4 |
|
80 |
58.8 |
|
70 |
8.8 |
|
Primary MF |
61.8 |
|
Secondary MF |
38.2 |
|
DIPSS score |
|
|
Intermediate-1 risk |
5.9 |
|
Intermediate-2 risk |
58.9 |
|
High risk |
29.4 |
|
N/A |
5.9 |
|
JAK2 mutation |
|
|
Yes |
58.9 |
|
No |
29.4 |
|
N/A |
11.8 |
|
Pre-allo-HSCT spleen size |
|
|
≤20 cm |
58.8 |
|
>20 cm |
41.2 |
|
Pre-allo-HSCT splenic irradiation |
|
|
Yes |
44.1 |
|
No |
55.9 |
|
Pre-allo-HSCT marrow fibrosis |
|
|
Grade 1 |
14.7 |
|
Grade 2 |
23.5 |
|
Grade 3 |
61.8 |
|
Conditioning regimen |
|
|
MAC |
23.5 |
|
RIC |
64.7 |
|
NMA |
11.8 |
Results
The median follow-up for patients was 47 months. Median OS was not reached; however, the 1-year overall OS rate was 58.8% (95% confidence interval [Cl], 44–78)2 and both reduced intensity conditioning and myeloablative conditioning regimens yielded similar 1-year OS rates (Figure 1).
Figure 1. 1-year OS rates for conditioning regimens*
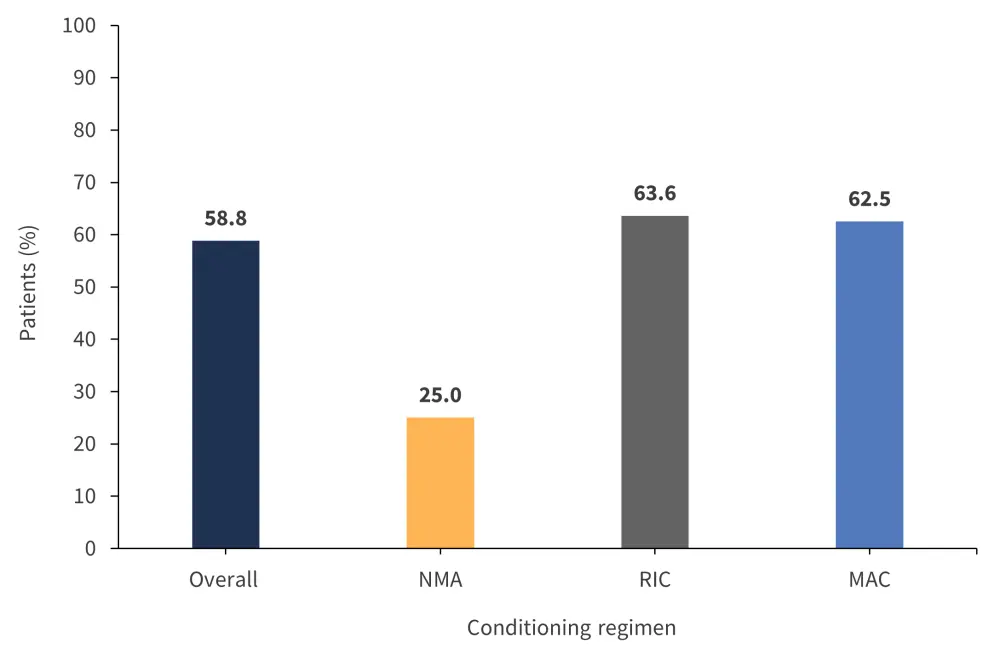
MAC, myeloablative conditioning; NMA, non-myeloablative conditioning; OS, overall survival; RIC, reduced-intensity conditioning.
*Adapted from Gandhi.1
- Neutrophil and platelet engraftment took a median of 17 and 31 days, respectively.
- Patients with a spleen size >20 cm and those undergoing splenic irradiation experienced delayed neutrophil engraftment (hazard ratio [HR], 0.44; p = 0.033; and HR, 0.37; p = 0.012, respectively) and delayed platelet engraftment (HR, 0.55; p = 0.153; and HR, 0.38; p = 0.026, respectively).
- The median dose of CD34 cells administered to patients was 5.9 × 106 cells/kg.2
- Higher CD34 cell doses were associated with faster neutrophil engraftment (HR, 1.42 per 106 cells; p < 0.001) but had no effect on platelet engraftment or OS.
- 19 patients experienced Grade ≥2 acute graft-versus-host disease (GvHD),2 with a median onset of 54 days after transplantation.
- 15 patients experienced chronic GvHD,2 with a median onset of 8 months after transplantation (Table 2).
Table 2. Rates of post-HSCT GvHD*
|
GvHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation. |
|
|
Characteristic, % |
N = 34 |
|---|---|
|
Acute GvHD |
|
|
None |
32.3 |
|
Grade 1 |
8.8 |
|
Grade 2 |
17.6 |
|
Grade 3–4 |
38.2 |
|
Engraftment syndrome |
2.9 |
|
Chronic GvHD |
|
|
None |
55.9 |
|
Limited |
14.7 |
|
Extensive |
26.5 |
|
Unknown extent |
2.9 |
Bone marrow fibrosis grade results were available in 24 patients. Over 1 year, 17 patients experienced bone marrow fibrosis regression, whereas 7 patients experienced none (Figure 2).1,2 Patients with Grade ≥2 acute GvHD were less likely to have bone marrow regression after 1 year (p = 0.292).
Figure 2. Number of patients experiencing bone marrow fibrosis regression in 1 year*
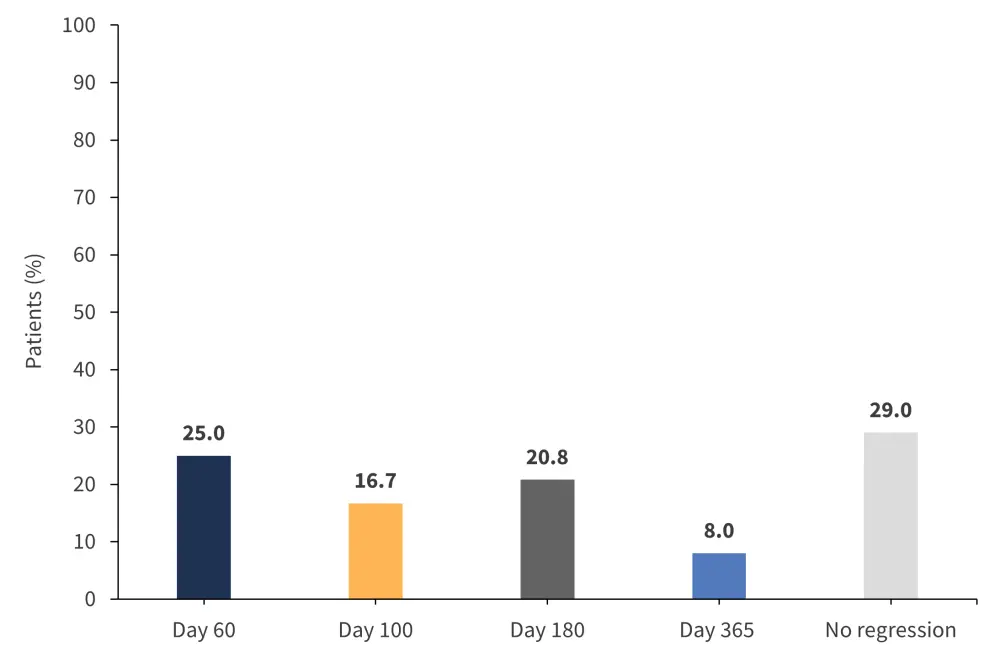
*Adapted from Gandhi.1,2
Predictors of any cause mortality were identified as:
- Bone marrow fibrosis regression (HR, 0.20; 95% CI, 0.04–1.08; p = 0.061)
- Grade ≥2 acute GvHD (HR, 4.42; 95% CI, 1.22–16.08; p = 0.024)
- Secondary MF (HR, 3.16; 95% CI, 1.16–8.61; p = 0.024)
Conclusion
The results from this study indicate a strong correlation between acute GvHD, lack of marrow fibrosis regression, and increased risk of patient mortality. Further investigation of the potential for novel treatment strategies such as T-cell depletion to rapidly remodel the bone marrow niche, thus improving OS in MF is warranted.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

