All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Bone marrow fibrosis (BMF) is a primary feature of myelofibrosis (MF) and is often associated with poor prognosis.1 While there are limited data on the association between BMF changes and clinical efficacy of MF treatments, improvement in fibrosis has shown evidence of disease modification.1
During the 64th American Society of Hematology (ASH) Annual Meeting and Exposition, Oh1 provided a presentation on the effects of ruxolitinib and momelotinib on BMF, as well as an analysis of the correlation between BMF changes and outcomes in Janus kinase (JAK) inhibitor-naïve patients with MF from the phase III SIMIPLIFY-1 study (NCT01969838). The SIMPLIFY-1 study design has previously been reported on the MPN Hub, and we are pleased to summarize the key findings presented by Oh1 below.
Results
At baseline, 58% of patients in both the momelotinib and ruxolitinib treatment groups had Grade 3 BMF. Moreover, when paired samples were analyzed, both momelotinib and ruxolitinib had similar effects on Grade ≥1 improvement in BMF (Figure 1).
Figure 1. Baseline grade improvements in momelotinib- and ruxolitinib-treated patients*
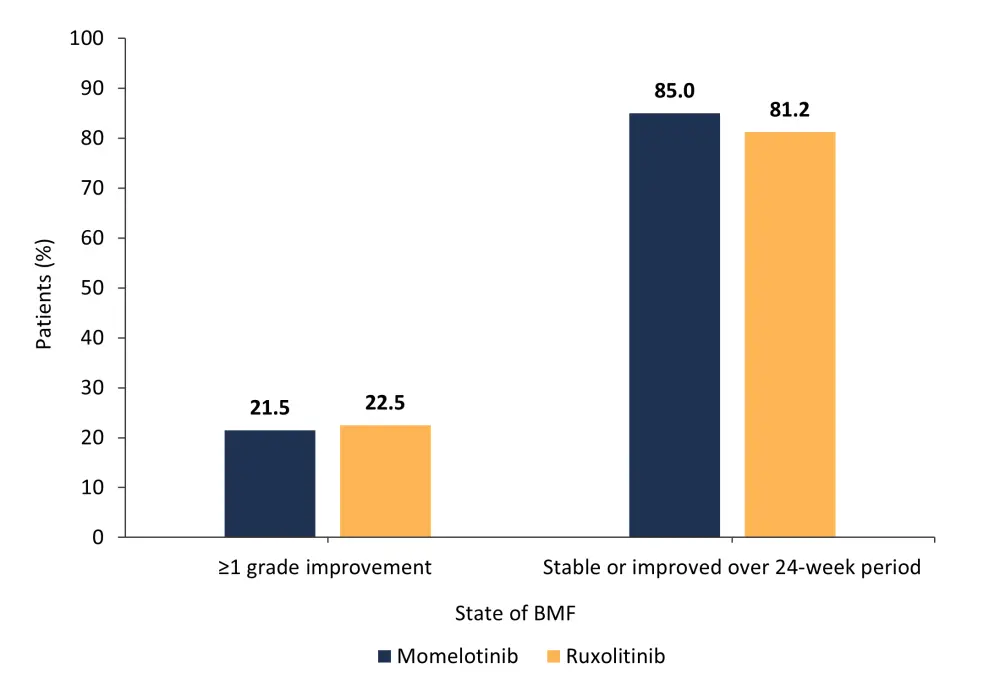
BMF, bone marrow fibrosis.
*Data from Oh.1
There was no association between BMF changes and Week 24 symptom responses or spleen responses for either momelotinib- or ruxolitinib-treated patients (Figure 2).
Figure 2. BMF changes and A) Week 24 symptom responses and B) spleen responses in momelotinib- and ruxolitinib-treated patients*
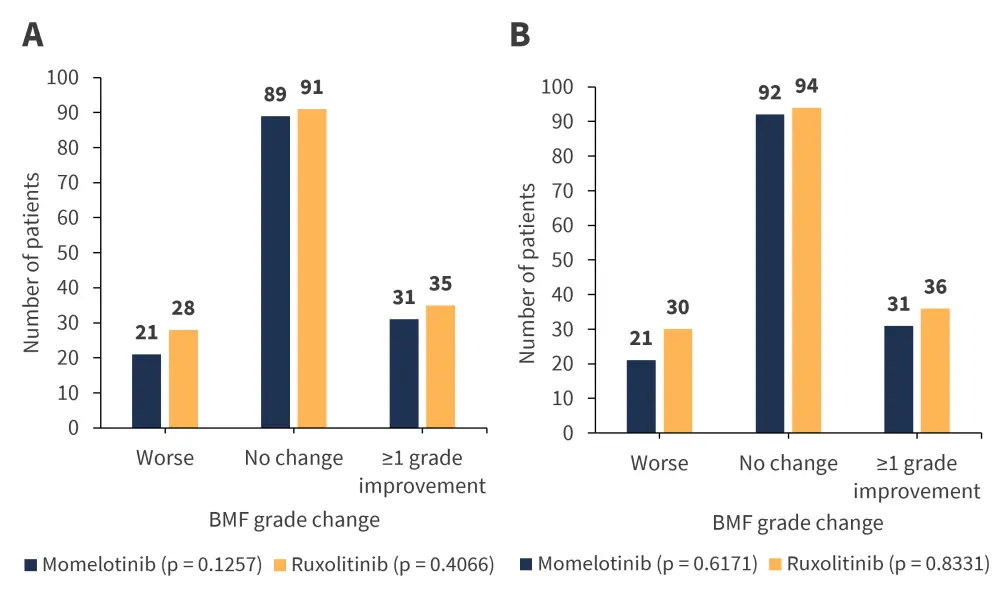
BMF, bone marrow fibrosis.
*Adapted from Oh.1
A total of 78% of patients treated with momelotinib had a Week 24 transfusion independence (TI) response (p = 0.3503), which was defined as the absence of red blood cell transfusions and no hemoglobin levels <8 g/dL in the 12 weeks before Week 12. In comparison, 53% of patients treated with ruxolitinib had a Week 24 TI response (p = 0.0963). Similar TI responses were recorded in both treatment groups with worsening BMF. In contrast, hemoglobin concentrations increased during momelotinib treatment, but decreased during ruxolitinib treatment despite worsening of BMF (Figure 3). Changes in BMF were also not associated with overall survival.
Figure 3. Average hemoglobin concentrations at baseline and Week 24 for patients treated with momelotinib and ruxolitinib*
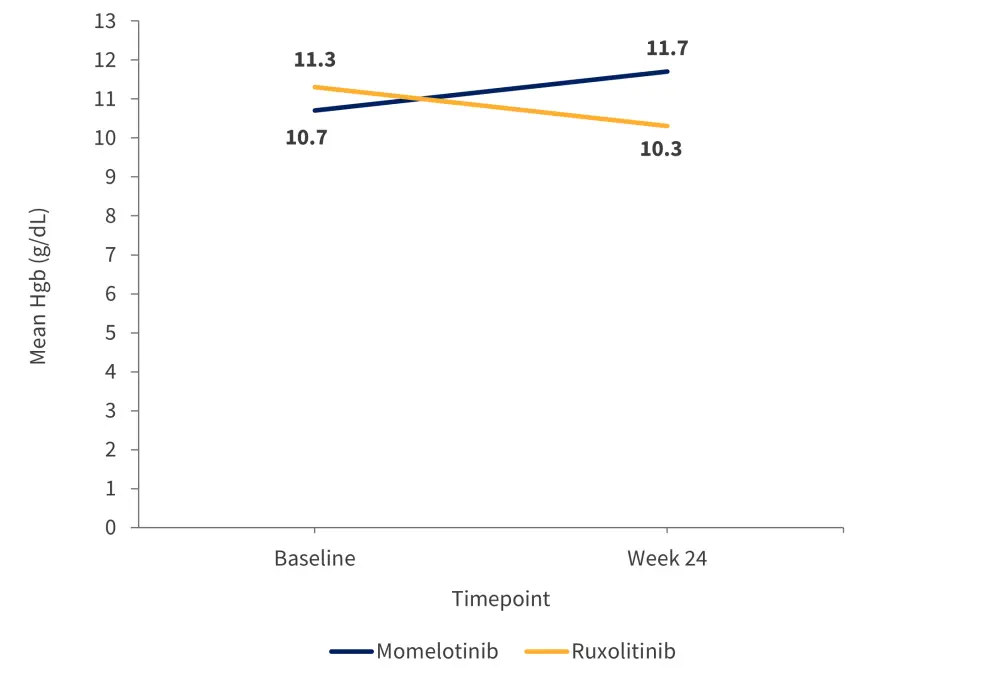
Hgb, hemoglobin.
*Adapted from Oh.1
Conclusion
In conclusion, BMF changes at Week 24 did not correlate with symptom or spleen response, anemia improvement, or overall survival in patients treated with momelotinib or ruxolitinib. However, 20% of patients who were JAK inhibitor naïve had a ≥1 grade BMF improvement within 24 weeks when treated with either drug. Collectively, these results highlight the need for better understanding of BMF changes by Week 24 of treatment and whether bone marrow assessment at Week 24 is of clinical benefit.
Expert Opinion
“I think there are many limitations on bone marrow fibrosis as an endpoint in studies, mainly with the validity of the scoring together with its limited levels,” said Dr Mesa, Executive Director of Atrium Health Wake Forest Baptist Comprehensive Cancer Center and co-author of the SIMPLIFY-1 study. “Medical therapies up to this point have not made a dramatic difference at a high level to fibrosis, and so there is not much surprise that there is no strong correlation between other markers of response and fibrosis.”
 Ruben A. Mesa
Ruben A. MesaReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

