All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Essential thrombocythemia with platelet count of 1.5 million
Extreme thrombocytosis (ExT), characterized by a platelet count of ≥1,500 × 109/L, is considered a risk factor for bleeding in patients with essential thrombocythemia (ET), and informs treatment decisions; read more here.1 Current guidelines recommend cytoreductive therapy in patients with ET and extreme thrombocytosis; however, there are inconsistencies on management of low-risk ET with extreme thrombocytosis due to concerns of greater risk for thrombosis and/or bleeding.2
Naseema Gangat et al.2 conducted a retrospective study using a threshold of platelet count ≥1,500 × 109/L to define extreme thrombocytosis and estimate the prevalence of extreme thrombocytosis at the time of ET diagnosis, characterize the phenotype and genotype of patients with extreme thrombocytosis, and to determine the impact on thrombotic and bleeding events.2 The results were recently published in Blood Advances2 and presented at the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition.3
For more information on how extreme thrombocytosis can affect the risk of thrombotic events in patients with low-risk ET, and specific therapy for these patients, see here.
Study design
A total of 710 patients with ET, evaluated between 1967–2021, were selected. The cohort was retrospectively reviewed according to the 2016 World Health Organization (WHO) diagnostic criteria. Cases with anemia defined by sex-adjusted hemoglobin levels (<11 g/dL in women, and <12.5 g/dL in men) were excluded to minimize the inclusion of patients with prefibrotic myelofibrosis. All cases were molecularly annotated for driver mutations with major arterial and venous thrombosis, and major hemorrhage defined by conventional criteria.
The reason behind the cutoff at ≥1,500 × 109/L in platelet count was the unique clinical characteristics of this subset of patients when compared with those with 1 and 1.49 million platelets:
- Younger age (40 vs 69 years; p < 0.0001)
- Predominance of female patients (73% vs 57%; p = 0.07)
- Predominance of CALR genotype (44% vs 28%; p = 0.02)
- Lower incidence of cardiovascular risk factors at/prior to diagnosis (35% vs 76%; p < 0.0001)
- Lower incidence of arterial thrombosis at/prior to diagnosis (7% vs 21%; p = 0.04)
- Higher rate of hemorrhage at/prior to diagnosis (15% vs 5%; p = 0.07)
Results
Out of the total cohort, 41 patients were identified to have extreme thrombocytosis. Table 1 shows the comparison of clinical and laboratory characteristics of patients with and without extreme thrombocytosis.
Table 1. Clinical and laboratory characteristics of patients*
|
ET, essential thrombocythemia; IPSET, International Prognostic Score for Thrombosis in ET. |
||
|
Characteristic |
Platelet count ≥1,500 × 109/L at diagnosis |
Platelet count <1,500 × 109/L at diagnosis |
|---|---|---|
|
Age, years, median (range) |
40 (19–86) |
59 (18–90) |
|
>60 years, % |
20 |
48 |
|
Female, % |
73 |
63 |
|
Hemoglobin, g/dL, median (range) |
13.1 |
13.9 |
|
Leukocyte count, 109/L, median (range) |
10.4 |
8.5 |
|
≥11 × 109/L, % |
43 |
21 |
|
Cardiovascular risk factors, % |
35 |
54 |
|
Driver mutation status, % |
||
|
JAK2 V617F |
34 |
62 |
|
CALR |
44 |
26 |
|
Major thrombosis at or prior to diagnosis, % |
||
|
Arterial |
7 |
14 |
|
Venous |
12 |
10 |
|
Major hemorrhage at or prior to diagnosis, % |
15 |
4 |
|
Microvascular symptoms, % |
23 |
24 |
|
Revised IPSET-thrombosis, % |
||
|
Very low |
46 |
21 |
|
Low |
20 |
22 |
|
Intermediate |
12 |
12 |
|
High |
22 |
45 |
|
Treatment at diagnosis, % |
||
|
Aspirin |
61 |
81 |
|
Cytoreductive therapy |
68 |
48 |
Prevalence of extreme thrombocytosis
Incidence rates of extreme thrombocytosis according to patient age were as follows:
- <40 years: 15% (20/133; p < 0.0001)
- 41–59 years: 5% (13/247; p < 0.0001)
- ≥60 years: 2% (8/330; p < 0.0001)
Incidence rate according to conventional ‘low-’ and ‘high-risk’ groups based on age >60 years and thrombosis history, was also reported:
- Low risk: 9% (27/315; p = 0.004)
- High risk: 4% (14/395; p = 0.004)
Characteristics of patients with extreme thrombocytosis
Univariate analysis of the phenotypic and genotypic characteristics of the 41 patients with extreme thrombocytosis demonstrated significant differences compared with those without extreme thrombocytosis:
- More likely to be younger (median age 40 vs 59 years; p < 0.0001)
- More likely to display CALR mutations (44% vs 26%; p = 0.001)
- Lower hemoglobin levels (median 13.1 vs 13.9 g/dL; p = 0.001)
- Leukocytosis ≥11 × 109/L (43% vs 21%; p = 0.001)
- Major hemorrhage at presentation (15% vs 4%; p = 0.001)
Transformation to myelofibrosis was identified in 24% of patients with extreme thrombocytosis at diagnosis compared to 13% of patients without extreme thrombocytosis (p = 0.05); this difference was thought to be due to a longer follow-up of patients with extreme thrombocytosis (11.2 vs 8.5 years; p < 0.001).
Next-generation sequencing (NGS) was conducted in 244 patients taken from the total cohort. A higher prevalence of gene mutations was observed among those diagnosed with extreme thrombocytosis, for the following genes:
- U2AF1 (7% vs 0.4%; p = 0.01)
- EZH2 (7% vs 0.9%; p = 0.04)
Impact on thrombotic and bleeding events
No significant associations were found for incidence rates of arterial thrombosis (7% vs 14%; p = 0.25) or venous thrombosis (12% vs 10%; p = 0.58), or microvascular symptoms (23% vs 24%; p = 0.87), between patients with and without extreme thrombocytosis, respectively.
Multivariable analysis for events at/prior to diagnosis identified several factors associated with both arterial and venous thrombosis:
- Arterial thrombosis
- male gender (odds ratio [OR], 1.8; p = 0.01)
- JAK2 mutation (OR, 2.4; p = 0.01)
- cardiovascular risk factors (OR, 2.4; p = 0.01)
- Venous thrombosis
- JAK2 mutation (OR, 2.7; p = 0.01)
Treatment details in low-risk patients with extreme thrombocytosis
Different treatment regimens were administered to 24 ‘low-risk’ patients with extreme thrombocytosis (Figure 1).
Figure 1. Treatment regimens for low-risk patients (n = 24) with extreme thrombocytosis*
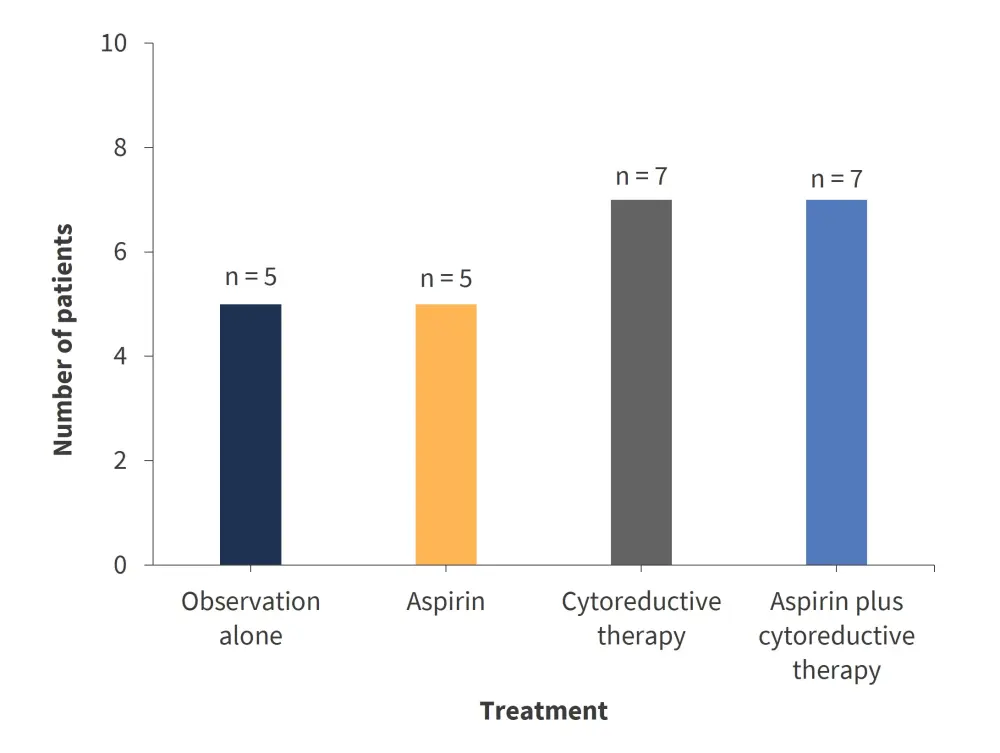
*Adapted from Gangat et al.2
At a median follow-up of 15.3 years, two arterial thrombotic events were documented with cytoreductive therapy that was ongoing at the time. A single venous thrombotic event was recorded post-diagnosis in a patient who was under observation. Of the patients on aspirin at diagnosis (n = 12), none experienced thrombotic events. The three events that occurred during this time were in absence of treatment, therefore, suggesting a protective effect of aspirin for both arterial and venous thrombosis.
Conclusion
The study2 identifies the genotypic and phenotypic characteristics of patients with ET and extreme thrombocytosis at diagnosis. Although the simplification of treatment options in patients with ET and extreme thrombocytosis is disputed by the very low frequency of informative cases, results from the study2 support current approaches of avoiding cytoreductive therapy in otherwise low-risk patients. Further evaluation in prospective, multi-center studies is warranted.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

