All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Polycythemia vera and essential thrombocythemia: 2021 updates
In a recent publication in the American Journal of Hematology, Tefferi and Barbui1 provided a review of the 2021 updates on the diagnosis, risk‐stratification, and management of polycythemia vera (PV) and essential thrombocythemia (ET). We hereby provide a summary of these updates and further insights into the two diseases.
Disease overview and diagnosis
PV and ET are classified as myeloproliferative neoplasms (MPN) and are caused by the excessive proliferation of bone marrow stem cells. PV is the most common MPN and is characterized by erythrocytosis and blood hyperviscosity. With only few exceptions, patients with PV harbor a Janus kinase 2 (JAK2) mutation either on exon 14 (JAK2V617F; 96%) or less commonly on exon 12 (3%). By contrast, mutations in the calreticulin gene (CALR) and the thrombopoietin receptor (MPL) are rarely seen in patients with PV.1,2 ET accounts for 25% of MPN and is defined by persistent thrombocytosis and megakaryocyte hyperplasia. Approximately 50–60% of patients with ET harbor the JAK2V617F mutation1,2 while aberrations in CALR and MPL are less common, with incidence rates of 25% and 3–5%, respectively. The most frequent MPL mutation in ET is MPLW515L/K.1,2 Nevertheless, a significant proportion of patents with ET do not express any of these three mutations (triple negative; 10–20%). Both diseases are often asymptomatic and predispose to thrombosis and hemorrhagic events. PV and ET diagnoses are performed based on the 2016 World Health Organization (WHO) criteria, shown in Table 1.3 It is worth noting that there are other MPN types that may display many characteristics of ET and that require additional information for accurate diagnosis. To distinguish ET from prefibrotic primary myelofibrosis (pre-PMF) or myelodysplastic/MPN with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T), bone marrow biopsies and peripheral blood smears are required. These help detect early signs of bone marrow fibrosis, as well as abnormal levels of erythrocytes, megakaryocyte progenitors, and leukoerythroblasts.
Table 1. WHO revised criteria for PV and ET diagnosis (2016)1,3
|
PV |
ET |
|---|---|
|
*BM biopsy might not be necessary if hemoglobin > 18.5 g/dL (hematocrit, 55.5%) in men or > 16.5 g/dL (hematocrit, 49.5%) in women. |
|
|
Diagnosis requires all three major criteria or the first two major and the minor criterion. |
Diagnosis requires all four major criteria or the first three major and the minor criterion. |
|
Major criteria 1. Hemoglobin: 2. BM tri-lineage proliferation with pleomorphic mature megakaryocytes* 3. Presence of JAK2 mutation (JAK2V617F or JAK2 exon 12) |
Major criteria 1. Platelets ≥ 450 × 109/L 2. BM megakaryocyte proliferation and formation of loose clusters 3. Not meeting WHO criteria for other myeloid neoplasms 4. JAK2/CALR/MPL mutated |
|
Minor criterion Subnormal serum erythropoietin level |
Minor criterion Presence of a clonal marker or absence of evidence for reactive thrombocytosis |
Risk factors
The approximate median survival for the general patient population with PV and ET has been reported as 14 years and 20 years, respectively, and 33 years and 24 years, respectively, for patients < 60 years.1 Nevertheless, various prognostic factors have been identified that affect survival by increasing the risk of death. In both PV and ET, these include age (> 60 years), leukocytosis, and history of thrombosis. Moreover, within 20 years, < 10% of patients with PV and approximately 5% of patients with ET will progress to acute myeloid leukemia.2 Progression to myelofibrosis is higher, with 10–20%2 of patients with PV and 4–11%4 of patients with ET.
Multiple large-scale studies have identified various risk factors that shorten survival or increase the risk for thrombosis, leukemic, or fibrotic transformation.1 All the identified risk factors for both PV and ET are depicted in Table 2. Traditionally, the risk stratification in PV and ET has focused on the likelihood of thrombotic events. Depending on the age and history of thrombosis, there are two risk categories for PV (low and high) and four categories for ET that also take into consideration the JAK2 mutational status (very low, low, intermediate, high) (Table 2 and Figures 1, 2 ).1 The CALR mutational status was previously considered for risk classification in ET but recent data have shown that it is associated with a lower incidence of thrombotic events without affecting the International Prognostic Scoring System.1 Recent studies have identified specific risk factors for arterial and venous thrombosis. In patients with ET, arterial thrombosis predictors are age (> 60 years), thrombosis history, cardiovascular risk factors, leukocytosis (> 11 × 109/L), and the presence of JAK2V617F. Interestingly, male gender has been identified as a predictor of venous thrombosis in ET.1 In patients with PV, history of hypertension predicted arterial thrombosis, and age (> 60 years) was a predictor of venous thrombosis. These new considerations should be taken into account when classifying patients into risk categories and should aid with refining the risk-adapted treatment plans.
Table 2. Identified risk factors in PV and ET1
|
|
PV |
ET |
|---|---|---|
|
*Identified adverse mutations/variants in PV: ASXL1, SRSF2, IDH2. |
||
|
Risk factors for: |
|
|
|
Survival |
Advanced age (> 60 years) |
Advanced age (> 60 years) |
|
Leukemic transformation |
Advanced age (> 60 years) |
Thrombosis history |
|
Fibrotic transformation |
JAK2V617F allelic burden > 50% |
Advanced age |
|
Thrombosis and bleeding |
Advanced age (> 60 years) |
Advanced age (> 60 years) |
|
Protective factors for: |
|
|
|
Fibrotic transformation |
— |
JAK2V617F mutation |
Risk-adapted treatment algorithms
The latest risk-adapted treatment algorithms proposed by Tefferi and Barbui for patients with PV and ET are discussed below. The risk definitions used by the authors are shown in Table 3.
So far, drug-targeted therapy does not seem to improve survival or prevent leukemic or fibrotic progression in PV or ET. Thus, therapeutic agents are targeted at minimizing and preventing the risk of thrombosis.1
Table 3. Risk classifications for PV and ET (Tefferi and Barbui, 2020)1
|
|
Very low risk |
Low risk |
Intermediate risk |
High risk |
|---|---|---|---|---|
|
ET, essential thrombocythemia; PV, polycythemia vera. |
||||
|
PV |
— |
No history of thrombosis, age ≤ 60 years |
— |
History of thrombosis or age > 60 years |
|
ET |
No history of thrombosis, age ≤ 60 years, JAK2 unmutated |
No history of thrombosis, age ≤ 60 years, JAK2 mutated |
No history of thrombosis, age > 60 years, JAK2 unmutated |
History of thrombosis or age > 60 years with JAK2 mutation |
Low-risk PV or ET
Cytoreductive therapy is not recommended for patients with low-risk ET or PV, and neither is pegylated interferon therapy for those with low-risk PV.
Low-dose aspirin has been repeatedly reported as beneficial for all risk categories in PV and for preventing venous thrombosis in JAK2V617-mutated low-risk ET. It has also been shown to reduce the risk of arterial thrombosis in patients with ET with concurrent cardiovascular risk factors. Low-dose aspirin appears to help reduce ET-associated vasomotor disturbances that arise from abnormal platelet–endothelial microvascular interactions. Such disturbances include headaches, lightheadedness, transient neurologic or ocular issues, tinnitus, paresthesia, atypical chest discomfort, and erythromelalgia. Administration of low-dose aspirin twice daily seems to be more effective than once daily for both PV and ET and should be considered for higher risk patients. Nevertheless, controlled trials are needed to further confirm the clinical superiority of twice-daily aspirin.1 In aspirin-refractory patients, platelet-reducing agents like hydroxyurea may be considered.
Moreover, there is substantial evidence of the beneficial role of phlebotomy to regulate hematocrit levels < 45%, in all patients with PV.1
In patients with PV-associated pruritus, management should initially involve non-pharmacological measures, like avoiding precipitating conditions, dry skin, and temperature-controlled baths. A recent study reported that the selective serotonin re-uptake inhibitor, paroxetine, can lead to > 50% responses for PV-associated pruritus (20 mg daily). Other drug-related therapeutics for PV-associated pruritus that have shown efficacy are JAK inhibitors, interferon α (IFN-α), and narrow-band UV-B phototherapy.1
In ET with concomitant presence of extreme thrombocytosis or acquired von Willebrand disease, aspirin is contraindicated as it increases the risk of hemorrhagic events.1
The recommended risk-adapted treatment algorithms for low-risk PV and ET are shown in Figure 1 and Figure 2, respectively.
Figure 1. Recommended treatment algorithm for PV1
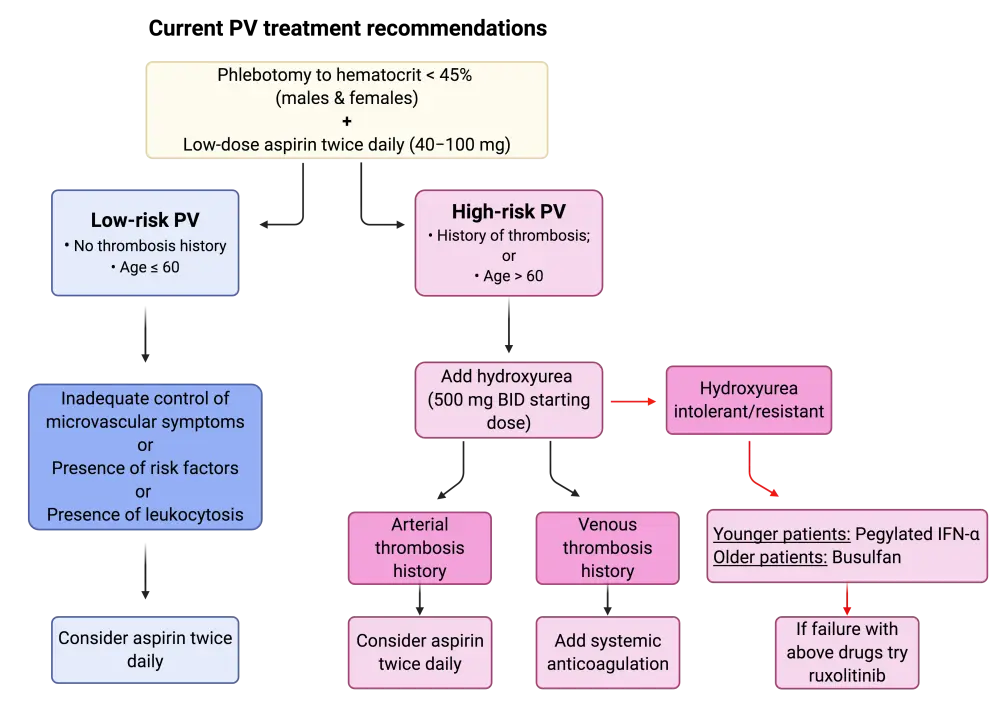
BID, twice daily; INF-α, interferon-α; PV, polycythemia vera.
Figure 2. Recommended treatment algorithm for ET1
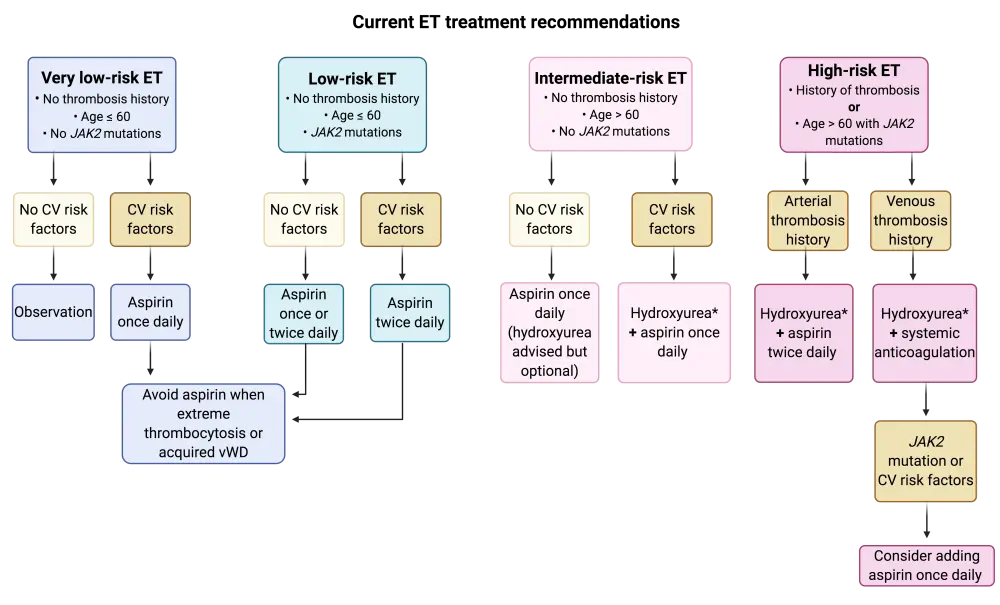
*In hydroxyurea refractory or intolerant patients, consider second line with pegylated INF-α (preferred) or busulfan.
CV, cardiovascular; ET, essential thrombocythemia; vWD, von Willebrand’s disease.
High-risk PV or ET
Following a plethora of studies, the current standard of care for high-risk patients with PV or ET has been identified as cytoreduction with hydroxyurea. There have been multiple trials comparing novel agents to hydroxyurea, but none have been deemed superior to date. As second line for high-risk ET or PV, pegylated interferon-α (INF-α) and busulfan have both shown good efficacy.1 The efficacy of pegylated INF-α therapy as frontline treatment of PV and ET has gathered a lot of clinical interest. The results of the first head-to-head phase III trial comparing hydroxyurea to ropepeginterferon α-2β as frontline treatment for PV revealed no differences in efficacy during the first year of treatment. Nevertheless, longer follow ups are needed to validate these results. For more information on this trial, read here.
For hydroxyurea-refractory or intolerant patients, there is a lack of randomized controlled trials for comparison of the current second-line treatments (INF-α, busulfan) with novel agents. Ruxolitinib, a JAK1/2 inhibitor, has been compared to hydroxyurea in this patient subset and has shown superior activity regarding the control of hematocrit, splenomegaly, and other symptoms. Nevertheless, no data were reported from this study regarding the effect of ruxolitinib on thrombosis or transformation prevention. According to the authors, there is currently no supporting evidence to justify the use of ruxolitinib in hydroxyurea-refractory/intolerant patients with PV. Ruxolitinib should be considered only in patients who fail to respond to hydroxyurea, INF-α, busulfan, and in the presence of severe symptomatology.1 Nevertheless, the results of the phase III RESPONSE trial have indicated that ruxolitinib continues to be superior to best available therapy (including INFs), even 5 years after treatment in patients with hydroxyurea-resistant/intolerant PV. Read more here.
As mentioned above, low-dose aspirin has shown beneficial effects in all patients with PV and ET, irrespective of risk category. Thus, its use is recommended in combination with the above drugs of choice.
The authors highlight the importance of avoiding agents that have shown suboptimal efficacy and/or safety and that are not supported by evidence-based research in PV or ET. Such drugs include chlorambucil, radiophosphorus, pipobroman, and anagrelide. These agents should be avoided as they have the potential to increase the risk of transformation and shorten patient survival.
Conclusion
The overarching aim of therapy for ET and PV is to prevent thrombohemorrhagic events and to provide the best possible symptom control. In patients with low-risk ET, cytoreductive agents are not recommended and observation or low-dose aspirin seems to suffice. In low-risk patients with PV, phlebotomy for hematocrit control together with low-dose aspirin once or twice daily, dependent on the presence of risk factors, is the standard of care. For high-risk patients with ET or PV, cytoreductive therapy with frontline hydroxyurea and second-line INF-α or busulfan are the agents of choice. Despite the rapid advances in the field, further clinical evidence is needed to validate the potential role of pegylated IFN-α therapy as frontline treatment for ET and PV before it can replace hydroxyurea. For more details on the available data on INF-α, read here.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

