All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Event-free survival following treatment with ropeginterferon alfa-2b in patients with PV
Ropeginterferon alfa-2b is a mono-pegylated proline interferon with disease-modifying properties, investigated as part of the PROUD-PV trial (NCT01949805) and its extension trial CONTINUATION-PV (NCT02218047). These trials aimed to establish the efficacy of ropeginterferon alfa-2b in reducing the risk of progression to myelofibrosis (MF) in patients with polycythemia vera (PV).1
The MPN Hub is pleased to summarize the final 6-year data from PROUD-PV and CONTINUATION-PV, with a focus on event-free survival and safety outcomes.
Study Design1
The study design and final results of PROUD-PV trial, and interim data from CONTINUATION-PV trial, have been previously published by the MPN Hub.
Briefly, 127 patients from PROUD-PV were treated in each arm, of which 95 patients in the ropeginterferon alfa-2b arm and 74 in the control arm (hydroxyurea [HU] or best available therapy [BAT]) were enrolled into CONTINUATION-PV trial. The baseline characteristics of patients in each arm were comparable.
Following the conclusion of PROUD-PV, patients were assigned an individualized dose-titration of ropeginterferon alfa-2b, with a median cumulative 4-weekly dose of 499 μg. In the control arm, 87.8% of patients remained on HU with a median dose of 1000mg/day. At final data collection, all patients had been treated ≥6 years.
Results1
Event-free survival and risk
The primary endpoint of this trial was event-free survival (EFS) at 6 years. At data cut off, median EFS had not been reached. The probability of EFS and the number of risk events which occurred in each cohort are outlined in Figures 1 and 2.
Figure 1. Probability of EFS in the ropeginterferon alfa-26 and control arm*
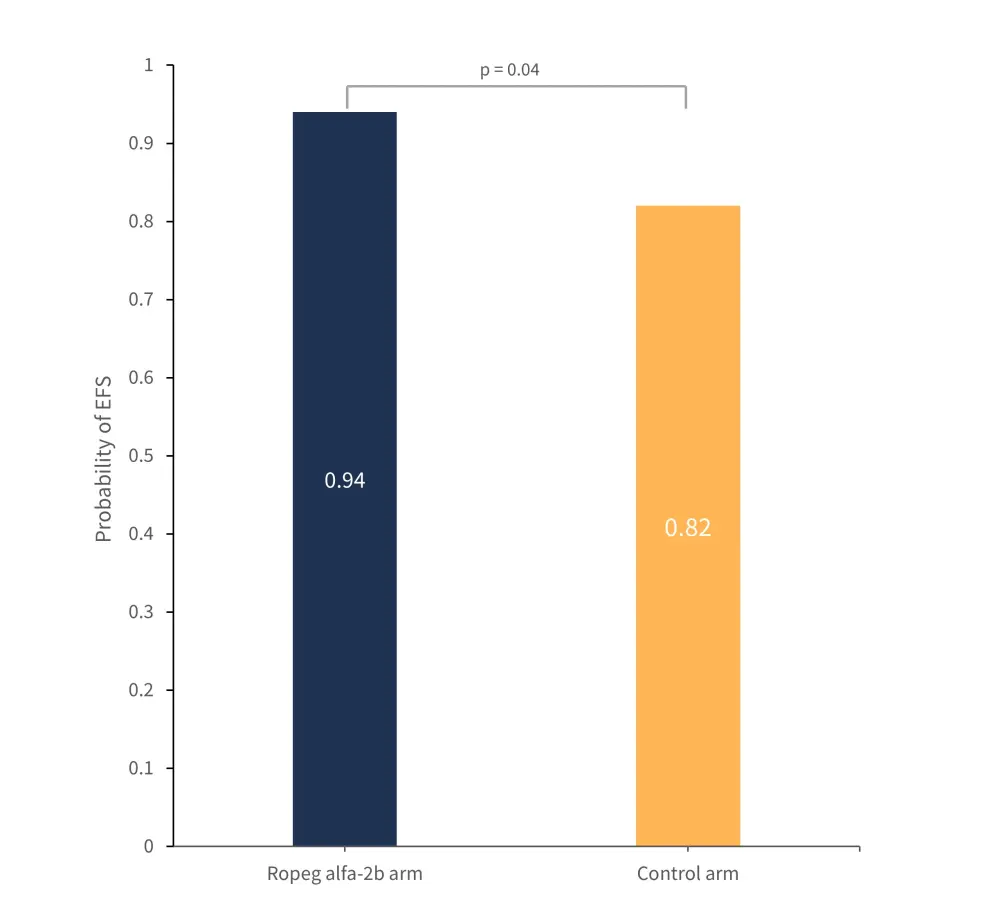
EFS, event-free survival; Ropeg alfa-26, ropeginterferon alfa-2b.
*Data from Gisslinger, et al.1
Figure 2. Number of risk events in the ropeginterferon alfa-2b and control arms*
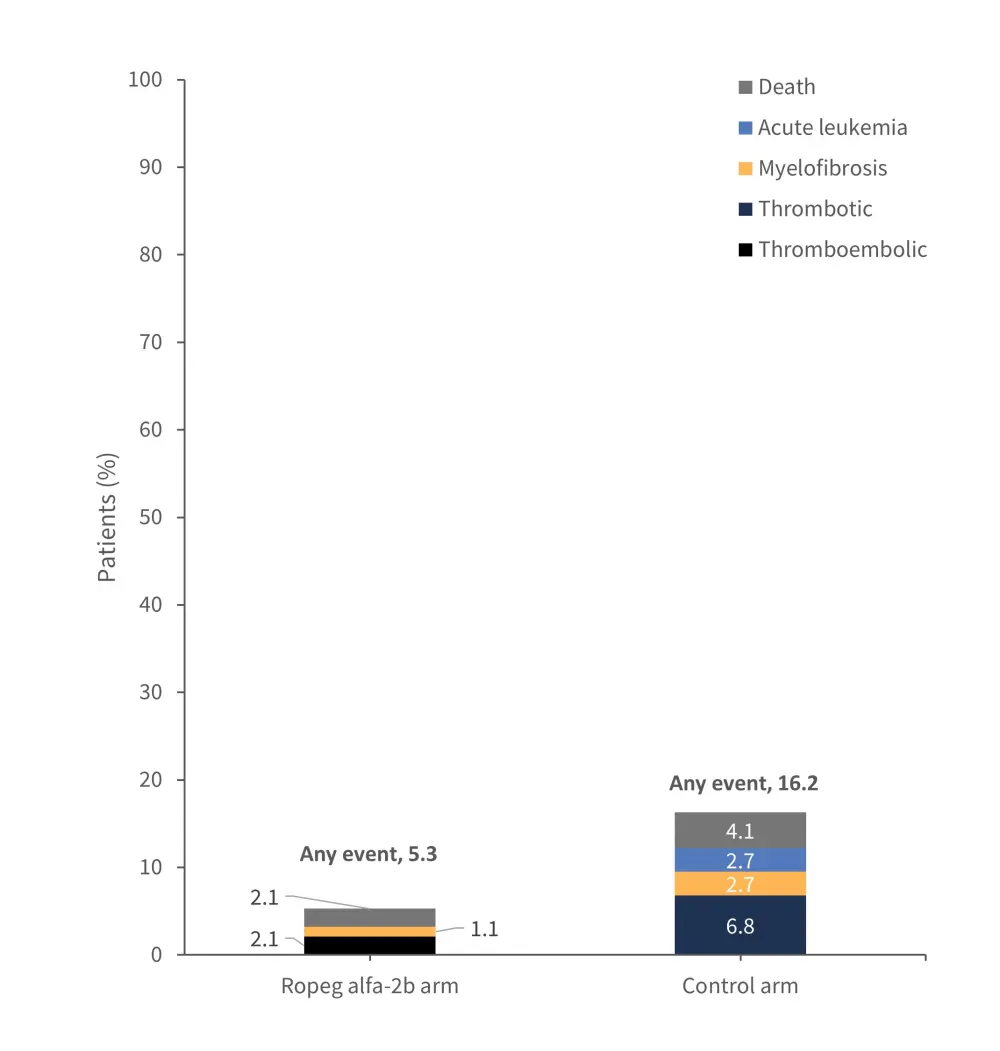
Ropeg alfa-26, ropeginterferon alfa-2b.
*Data from Gisslinger, et al.1
JAK2V617F allele burden
A surrogate endpoint of JAK2V617F allele burden was used as a measure of disease modification. In the ropeginterferon alfa-2b arm, a greater molecular response was observed and a smaller median JAK2V617F allele burden at 6-years (Figure 3).
Figure 3. Molecular response and median JAK2V617F allele burden at 6 years observed in the ropeginterferon alfa-2b and control arms*
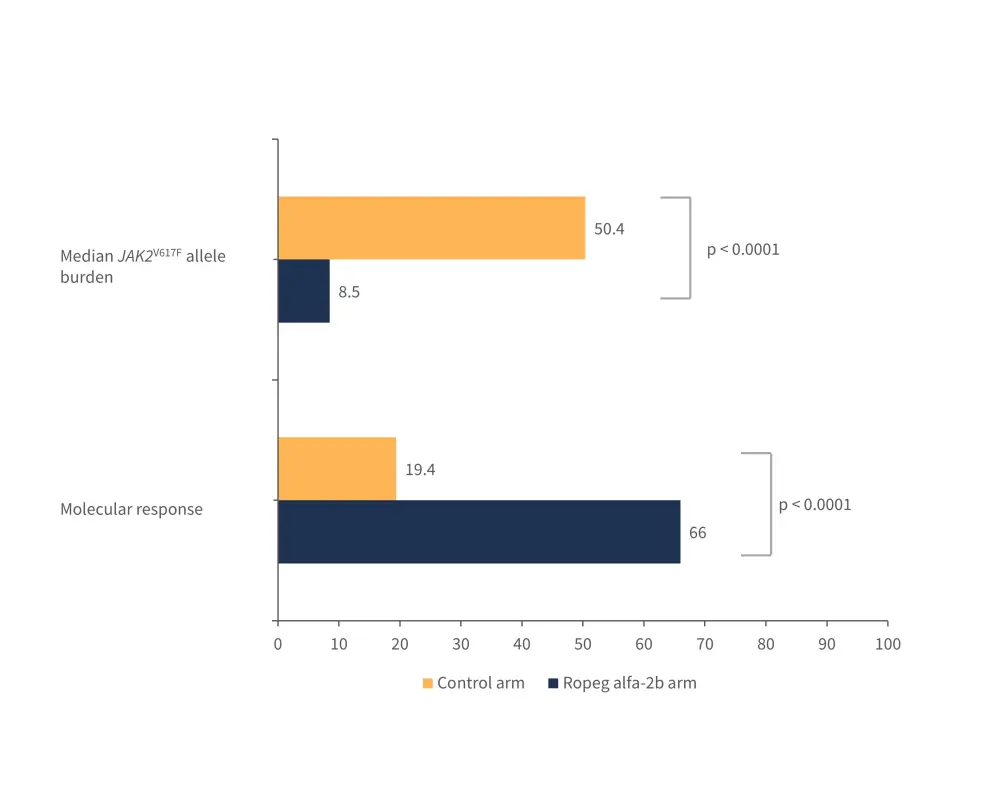
Ropeg alfa-26, ropeginterferon alfa-2b.
*Data from Gisslinger, et al.1
Complete hematologic response
The significantly high complete hematologic response (CHR) at 3-year persisted.
- At 72 months, CHR rate was higher in ropeginterferon alfa-2b compared with control arm (54.5% vs 34.9%, p = 0.02)
- Although the onset of CHR was more gradual in the ropeginterferon alfa-2b arm, the response was more durable compared with control arm (60.9% vs 41.2%, p = 0.04).
Safety1
Key safety outcomes are summarized in Table 1.
Table 1. Key safety outcomes*
|
BAT, best available therapy; HU, hydroxyurea; PV, polycythemia vera. |
||
|
Safety outcomes, % |
Ropeginterferon alfa-2b (n = 95) |
Control (HU or BAT) (n = 74) |
|---|---|---|
|
Discontinuation of therapy due to drug-related toxicity |
11.0 |
2.4 |
|
Grade ≥3 treatment related adverse events |
15.7 |
16.5 |
|
PV-associated adverse events |
7.1 |
12.1 |
Conclusion
Following treatment for ≥6 years, ropeginterferon alfa-2b resulted in an improved EFS compared with HU or BAT. Furthermore, a higher molecular response, accompanied by lower burden of the JAK2V617F allele was observed with ropeginterferon alfa-2b treatment. However, it is important to note that safety data was not consistently improved compared with the control arm, with a higher number of patients discontinuing treatment and no significant differences observed Grade ≥3 treatment related adverse events.
The long-term results from PROUD-PV and CONTINUATION-PV provide some evidence to support the disease modification properties of ropeginterferon alfa-2b and its application toward reducing the risk of disease progression in patients with PV.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




