All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
The U.S. Food and Drug Administration (FDA) has granted accelerated approval for pacritinib, a Janus kinase (JAK) and IRAK1 inhibitor, at a dose of 200 mg twice daily for the treatment of adult patients with intermediate- or high-risk primary or secondary myelofibrosis (MF) and platelet count <50 × 109/L based on results of the phase III PERSIST-2 trial (NCT02055781).1
Severe thrombocytopenia is associated with poor prognosis (i.e., greater risk of bleeding, symptom burden, leukemic transformation, and poor survival). The PERSIST-2 trial compared pacritinib (once daily or twice daily) and the best available therapy (ruxolitinib, 45%; hydroxyurea, 19%; prednisone and/or prednisolone, 13%; and watchful-waiting only, 19%) in 311 patients with MF and severe thrombocytopenia.
Efficacy analysis demonstrated that pacritinib arms (combined) were associated with:
- higher ≥35% spleen volume reduction (SVR) (18% vs 3% with best available therapy; p = 0.001)
- higher ≥50% reduction in total symptom score (TSS) (25% vs 14% with best available therapy; p = 0.08)
Pacritinib twice daily led to a significantly greater spleen and symptom response compared with the best available therapy. Red blood cell transfusion burden at 24 weeks was lower with pacritinib (Figure 1). In terms of safety, nonhematologic adverse events (AEs) were mild in severity; diarrhea was the most common AE observed with pacritinib (48% of patients on the 200 mg twice daily dose).2
Overall, pacritinib was found to be well tolerated with low-grade gastrointestinal AEs and rare treatment discontinuations, and more effective than the best available therapy in reducing symptom burden and spleen volume.2
Figure 1. Summary of the PERSIST-2 trial*
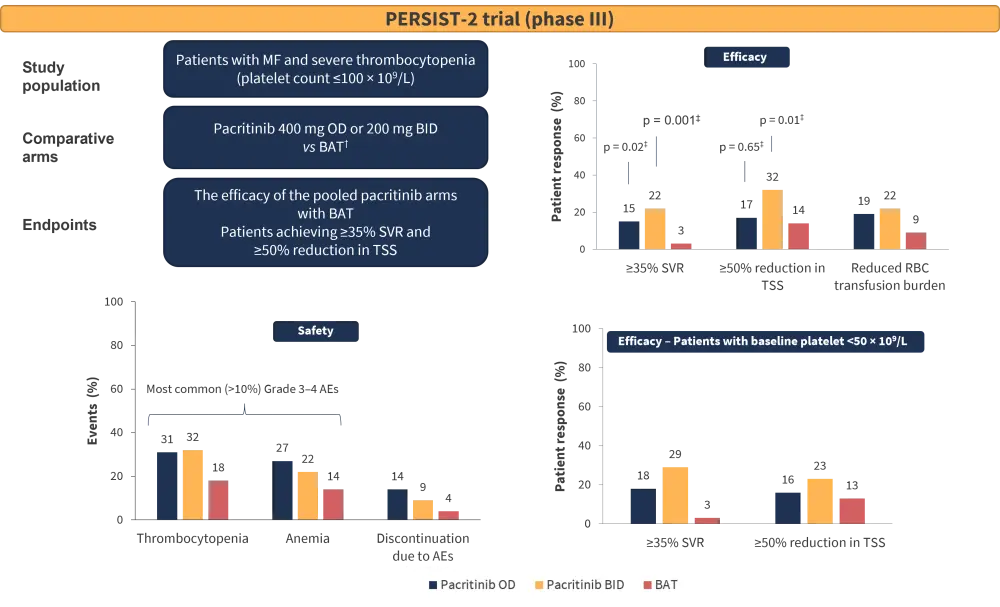
AEs, adverse events; BAT, best available therapy; BID, twice daily; MF, myelofibrosis; OD, once daily, RBC, red blood cell; SVR, spleen volume reduction; TSS, total symptom score.
*Adapted from Mascarenhas et al.2
†Allows ruxolitinib.
‡versus BAT.
The MPN Hub Steering Committee member John Mascarenhas, recently gave an interview after his presentation on the PERSIST-2 trial results at the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition.
How effective is pacritinib in managing symptoms and splenomegaly in cytopenic patients with MF?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 John Mascarenhas
John Mascarenhas