All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Polycythemia vera (PV) is a BCR-ABL1-negative subtype of myeloproliferative neoplasms (MPN), characterized by excessive erythroid progenitor and mature cell production. In the majority of cases, this is a result of a mutation in the Janus kinase 2 gene (JAK2 V617F). Givinostat, a histone-deacetylase inhibitor, selectively targets JAK2 V617F cell growth to reduce hematopoietic cell proliferation.
Three open-label 24-week phase I/II studies have reported that givinostat, either as monotherapy or in combination with hydroxyurea, gave a high response rate (50–80%) and had a good safety profile in patients with PV.1 Those who received clinical benefit from givinostat in these studies were able to enter a compassionate use program. Alessandro Rambaldi and colleagues conducted a long-term study (NCT01761968) of patients in the program, and here we present a summary of their 4-year findings, which were published in Blood Cancer Journal.1
Study design
Eligible patients were ≥18 years old, JAK2 V617F-positive with a diagnosis of MPN according to the World Health Organization criteria, and had tolerated previous givinostat treatment and achieved a clinical benefit at the end of one of the three open-label core studies and/or a compassionate use program. The core studies were:
- A pilot study in 29 patients with JAK2 V617F-positive MPN who were refractory to hydroxyurea, or were young and required cytoreductive therapy; patients received givinostat monotherapy at 50–150 mg/day2 (NCT00606307)
- A phase II study of 44 patients with JAK2 V617F-positive PV who were non-responders to hydroxyurea and received givinostat at 50–150mg/day alongside hydroxyurea at the maximum tolerated dose3 (NCT00928707)
- A dose-determination study of givinostat in 48 patients with JAK2 V617F-positive PV4 (NCT01901432)
Primary objectives of Rambaldi et al.’s study were determination of long-term safety and tolerability, and assessment of efficacy (complete response [CR] and partial response [PR] rate).
Patients received givinostat with or without concomitant hydroxyurea at the last tolerated dose and regimen as in the core study or compassionate use program. Dose adjustments to optimize individual responses were permitted.
Patients
In total, 78 patients with MPN across 15 centers in France, Germany, Italy, and the UK entered the compassionate use program; 53 of these subsequently enrolled in the long-term study, in addition to one patient who had not participated in any of the core studies. The majority of patients had PV (n = 51) and, at the cut-off date of December 31, 2018, 50 of these had received ≥1 dose of givinostat. Of these, 15 were treated with concomitant hydroxyurea. Baseline characteristics of the patients with PV are shown in Table 1. Median exposure to givinostat was 2.8 years (range, 3 months – 11 years). At data cut-off, 31 patients (62%) were ongoing in the study.
Table 1. Baseline characteristics for patients with PV treated with givinostat*
|
JAK, Janus kinase; PV, polycythemia vera; SD, standard deviation. |
|
|
Characteristic |
N = 50 |
|---|---|
|
Median age, years (range) |
59 (42–80) |
|
Male, % |
62 |
|
Race, White, % |
100 |
|
Mean time since diagnosis, years ± SD |
7.2 ± 5.7 |
|
Hematology, median (range) |
|
|
JAK2 V617F-positive, % |
100 |
|
Median JAK2 V617F allele burden, % (range) |
59.8 (25.0–94.2) |
|
Prior PV therapy, % |
|
|
Number of prior PV therapies, % |
|
Key findings
Safety
During the long-term study, 96% of patients experienced at least one adverse event (AE); however, 89.7% of these events were Grade <3. Overall, 64% of patients (n = 32) experienced treatment-related AEs (TRAEs) of any grade, the most common of which were blood and lymphatic system disorders (24%) and gastrointestinal disorders (32%). Five patients reported Grade 3 TRAEs, including thrombocytopenia, diarrhea, asthenia, QTc (corrected QT interval) prolongation, and hypertension (n = 1 each), and there were no Grade 4 or 5 events. Treatment-related QTc prolongation was noted in three patients, one case of which was Grade 3 and resulted in treatment discontinuation. All patients who received concomitant hydroxyurea experienced at least one AE, including Grade 3 cases of thrombocytopenia and asthenia.
Efficacy
Over 80% patients had a CR or PR maintained for the duration of follow-up (Figure 1). Four patients (8%) were defined as non-responders on study entry, but they were enrolled as givinostat provided them with benefit from disease-related symptoms and/or hematological parameters. Interestingly, overall response was higher in patients treated with givinostat monotherapy (97%) compared to those who received concomitant hydroxyurea (80%). Moreover, a reduction in mean JAK2 V617F allele burden was observed at the majority of patients’ annual visits.
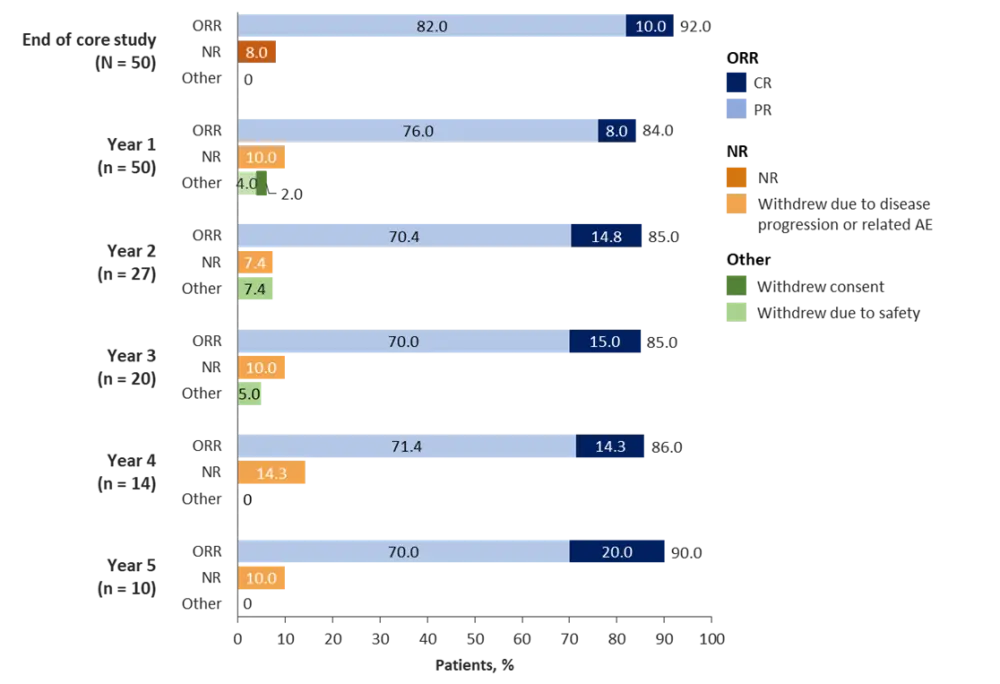
AE, adverse event; CR, complete response; NR, non-responder; ORR, overall response rate; PR, partial response.
Conclusion
The first interim analysis of this long-term study found that givinostat demonstrated a good safety and efficacy profile over 4 years, supporting its long-term use in patients with PV. Limitations of the study included its ongoing nature; thus patients were at different stages of treatment at the cut-off with full 4- and 5-year data unavailable for all patients. In addition, only patients who had previously achieved clinical benefit with givinostat were included, with no comparator arms.
One of the study authors, MPN Hub Steering Committee member Alessandro M. Vannucchi, discussed the findings below.
Expert Opinion
Current treatment of patients with polycythemia vera (PV) has significantly improved over the last years, following the understanding of the molecular basis of disease, characterized by an almost universal association with JAK2 mutation. There are currently two approved drugs for PV, the JAK1/JAK2 inhibitor ruxolitinib for patients resistant or intolerant to hydroxyurea, and ropeginterferon for those without splenomegaly in need of cytoreduction. Yet, the impact of these treatments on the natural history of PV remains unclear, and both suffer from side effects, leaving some clinical needs still unmet.
Givinostat is a histone-deacetylase inhibitor (a group of enzymes involved in DNA regulation) that is preferentially active against JAK2 V617F-mutated progenitor cells and proved able to control blood cell counts, reduce phlebotomy requirements or the needed dose of hydroxyurea, and improve symptoms, in 3 pilot studies in patients with PV, overall documenting remarkable safety profile. The study published by Alessandro Rambaldi and coworkers in Blood Cancer Journal reports on a cohort of 51 patients with PV who were treated with Givinostat for a mean of 4 years as extension phase from the 3 pilot studies. Results confirmed the substantial safety of Givinostat as well as its long-term efficacy, with greater than 80% of the patients having clinical and hematologic responses to the drug. These findings are particularly important as a randomized phase III study with givinostat is planned to start mid-2021, and results might hopefully lead to new therapeutic option for patients with PV
 Alessandro M. Vannucchi
Alessandro M. VannucchiReferences
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

