All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Hydroxyurea and secondary malignancies in older patients with MPN
Introduction
Hydroxyurea (HU) is commonly used for cytoreduction in the treatment of myeloproliferative neoplasms (MPN). Patients with MPN are at an increased risk of secondary malignancies (SM) such as acute myeloid leukemia and myelodysplastic syndromes.
While HU has demonstrated efficacy in the treatment of MPN, there are concerns that it may have mutagenic and leukemogenic potential due to its interference with DNA synthesis. Questions remain around any potential link between exposure to HU and the development of SM. Results from a large, United States (U.S), retrospective study assessing the link between HU and SM were recently published by Wang, et al., in Blood in 2022.1 Below, we summarize the key findings.
Methods
This study used data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database, resulting in a large retrospective cohort of patients with MPN from 2010 to 2017. Inclusion and exclusion criteria are shown in Figure 1.
Figure 1. Cohort selection*
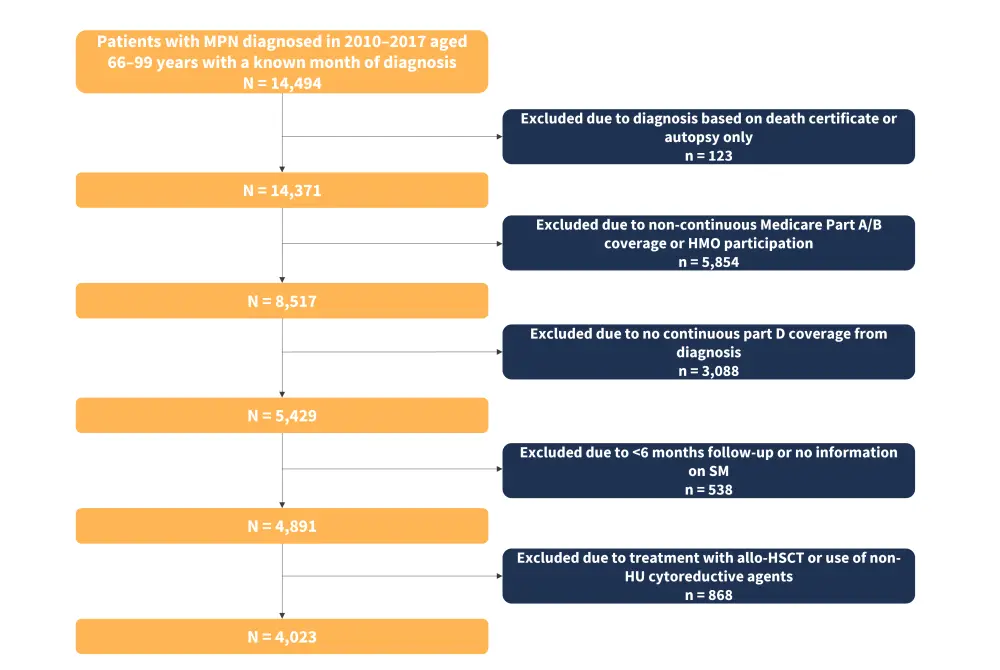
Allo-HSCT, allogeneic hematopoietic stem cell transplant; HMO, health maintenance organization; HU, hydroxyurea; MPN, myeloproliferative neoplasms; SM, secondary malignancy.
*Adapted from Wang, et al.1
Patients were followed from MPN diagnosis through to the diagnosis of a SM, death, ending of Medicare coverage, or end of the study. HU proportion of days covered (PDC) was calculated as the ratio of days the patient was covered by HU to days from HU initiation to 6 months before the end of follow-up. Patients who never received HU would have a PDC of 0%, and patients who received HU every day from treatment initiation through to 6 months before the end of follow-up would have a PDC of 100%.
The outcome of interest was any new malignancy, other than MPN and non-melanoma skin cancer, diagnosed at least 6 months after cohort entry.
Statistical analysis
The cumulative incidence function of SM was computed via a competing risk model, with death and the occurrence of SM considered competing events. Multivariable competing risk regression models were developed using the Fine and Gray method to estimate the adjusted hazard ratios (HRs) for SM. HU was first analyzed as a binary variable, i.e., comparison between users and non-users, then as a continuous variable with every 10% increase in PDC. HU treatment was investigated as a time-varying covariate with patients initially considered non-users and then users for the remainder of follow-up. HU was further analyzed for any link to a specific subtype of SM (solid, lymphoid, and myeloid), with other types of SM considered competing events for each specific subtype. Patient characteristics were adjusted for in the multivariable model.
Results
The final cohort included 4,023 patients, with 2,683 treated with HU after diagnosis and 1,340 not treated with HU (Table 1).
Table 1. Patient characteristics*
|
ET, essential thrombocythemia; IQR, interquartile range; MF, myelofibrosis; MPN, myeloproliferative neoplasms; PV, polycythemia vera; Q, quintile. |
|||
|
Characteristics, % (unless otherwise stated) |
Overall (N = 4,023) |
Hydroxyurea |
|
|---|---|---|---|
|
Yes (n = 2,683) |
No (n = 1,340) |
||
|
Type of MPN |
|
|
|
|
PV |
42 |
37.9 |
50.1 |
|
ET |
49.1 |
57.7 |
31.9 |
|
MF |
8.9 |
4.4 |
18 |
|
Median age (IQR), years |
77 (77–83) |
76 (71–83) |
77 (72–83) |
|
Female sex |
61.3 |
66 |
52 |
|
Comorbidity index |
|
|
|
|
0 |
12.6 |
12.8 |
12.1 |
|
1–2 |
40 |
42.5 |
34.9 |
|
≥3 |
47.4 |
44.7 |
53 |
|
Previous cancer |
|
|
|
|
Yes |
24 |
23.7 |
24.4 |
|
Disability |
|
|
|
|
Yes |
11.4 |
10.8 |
12.6 |
|
Yorst index† |
|
|
|
|
5th Q |
|
33.5 |
31.8 |
|
4th Q |
32.9 |
20.5 |
20.7 |
|
3rd Q |
20.6 |
15.8 |
16.6 |
|
2nd Q |
16.1 |
15.6 |
14.9 |
|
1st Q |
15.4 |
10 |
12.2 |
|
Unknown |
10.7 |
4.6 |
3.7 |
In the 4,023 total patient cohort, 12.2% of patients developed a SM.
- The median follow-up time was 3.25 years, with a longer follow-up time of 3.58 years versus 2.58 years among those who received HU treatment versus those who did not, respectively.
- The median time to develop a SM was 2.51 years (interquartile range [IQR], 1.49–4.39) for patients who received HU treatment and 1.84 years (IQR, 1.05–3.15) for patients who never received HU.
- The cumulative incidence probability of SM for patients who received HU treatment was 19.88% (95% confidence interval [CI], 17.16–22.75%) versus 22.31% (95% CI, 17.51–27.47%) for patients who didn’t receive HU treatment which was statistically significant (p < 0.01).
- However, in the multivariable competing risk model, post adjusting for patient characteristic variables, receiving HU treatment (HR, 0.92; 95% CI, 0.74–1; p = 0.49) or HU PDC (every 10% increase: HR, 1.01; 95% CI, 0.98–1.03; p = 0.43) were not significantly associated with the risk of SM.
A total of 8.6%, 1.8%, and 1.7% of patients developed a solid, lymphoid hematological, and myeloid hematological SM, respectively. The cumulative incidence probability of a solid SM was statistically significantly lower for patients treated with HU compared with patients who did not receive HU treatment (Table 2). The most common solid SMs were lung and bronchus cancer (n = 72), breast cancer (n = 49), and melanoma (n = 40). In the lymphoid hematologic SM subtype, non-Hodgkin lymphomas were most common (n = 31) and in the myeloid hematological SM subtype, acute myeloid leukemia (n = 41) and myelodysplastic syndromes (n = 15) were most common.
Table 2. Cumulative incidence function of secondary malignancies*
|
CI, confidence interval; HU, hydroxyurea; SM, secondary malignancies. |
|||||
|
Malignancy type |
HU use |
SM, n |
Incidence (95% CI per 1,000 person), years |
Cumulative incidence function (95% CI), % |
p value |
|---|---|---|---|---|---|
|
Solid SM |
HU user |
218 |
20.4 (17.9–23.3) |
14.95 (12.42–17.7) |
0.03 |
|
Non-HU user |
128 |
30.5 (25.7–36.3) |
15.25 (11.38–19.65) |
||
|
Lymphoid SM |
HU user |
43 |
4 (3–5.4) |
2.51 (1.78–3.45) |
0.1 |
|
Non-HU user |
30 |
7.2 (5–10.2) |
4.69 |
||
|
Myeloid SM |
HU user |
46 |
4.3 (3.2–5.7) |
2.42 (1.78–3.45) |
0.71 |
|
Non-HU user |
24 |
5.7 (3.8–8.5) |
2.36 (1.48–3.58) |
||
The multivariable competing risk model determined that risk of SM was not influenced by whether patients received HU or the HU PDC. HU treatment, or the percentage of days receiving HU, was not statistically significantly associated with incidence of SM or any individual subtype of SM (Table 3).
Table 3. Multivariable analysis of HU use and types of secondary malignancies*
|
AML, acute myeloid leukemia; CI, confidence interval; HU, hydroxyurea; MDS, myelodysplastic syndromes; PDC, proportion of days covered; SM, secondary malignancy. |
|||
|
HU as a binary variable |
|||
|---|---|---|---|
|
SM type |
Hazard ratio |
95% CI |
p value |
|
All SM |
0.99 |
0.82–1.2 |
0.92 |
|
Solid SM |
0.92 |
0.74–1.16 |
0.49 |
|
Lymphoid SM |
1.01 |
0.61–1.68 |
0.97 |
|
Myeloid SM |
1.5 |
0.91–2.46 |
0.11 |
|
AML/MDS |
1.33 |
0.77–2.29 |
0.3 |
|
HU PDC (every 10% increase) |
|||
|
All SM |
1.01 |
0.99–1.03 |
0.43 |
|
Solid SM |
1 |
0.98–1.03 |
0.73 |
|
Lymphoid SM |
0.99 |
0.94–1.05 |
0.8 |
|
Myeloid SM |
1.06 |
1–1.12 |
0.07 |
|
AML/MDS |
1.03 |
0.97–1.1 |
0.32 |
HU treatment was not determined to be associated with SM; however, several covariates were.
- Age at MPN diagnosis was a significant factor, with patients diagnosed at age ≥85 years less likely to develop a SM compared with patients diagnosed between 66–69 years of age (p < 0.01).
- Patients with a disability were significantly less likely to develop SM (p < 0.01) and solid SM (p = 0.02) than patients without.
- Patients aged ≥85 years at diagnosis and patients with a disability may have been shown to be less likely to develop a SM due to an increase in the competing risk of death in these groups.
- Male patients were significantly more likely to develop any SM (p = 0.04), lymphoid SM (p < 0.01), and myeloid SM (p = 0.01) than female patients.
- Patients in the 4th quintile of the Yorst index were more likely to develop solid SM (p = 0.03), and patients in the 3rd quintile of the Yorst index were more likely to develop a lymphoid SM (p = 0.03) than patients in the 5th quintile.
- Myeloid SMs were more likely in patients in the Midwest (p < 0.01) and West (p < 0.01) compared with patients in Northeast.
- Type of MPN also affected the likelihood of developing a myeloid SM.
- Patients with myelofibrosis were more likely to develop myeloid SM than patients with polycythemia vera (p < 0.01).
Conclusion
These results indicate that patients with MPN treated with HU did not have a significantly higher rate of SM than patients who were not treated with HU, with the risk of SMs not shown to be increased by HU use in older patients. Despite the large cohort, longer follow-up times are recommended to confirm these results. Also, the exclusion of non-melanoma skin cancers, the most common SM in MPN patients, suggests a need for further investigation.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


