All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
IMbark phase II study of imetelstat for R/R myelofibrosis: Updates from ASH 2020
Although Janus kinase (JAK) inhibition provides symptomatic relief for patients with myelofibrosis (MF), it does not induce long-term remission or alter disease course, and there is currently an unmet need for novel therapies to effectively target the disease at the level of the malignant stem cell. Telomerase is highly upregulated in malignant hematopoietic stem and progenitor cells and causes uncontrolled proliferation. Short telomere length and higher telomerase activity correlate with higher risk and shorter survival in patients with myeloid malignancies. Imetelstat is a first-in-class therapy which competitively inhibits the enzymatic activity of telomerase, thereby impeding the proliferation of malignant cells.
Here we summarize the results of the IMbark phase II trial (MYF2001; NCT02426086) of imetelstat for patients with MF who have relapsed after or are refractory to (R/R) JAK inhibition, including clinical efficacy and pharmacodynamic (PD) data. These were presented by MPN Hub Steering Committee member John Mascarenhas over three sessions during the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition.1,2,3
IMbark phase II trial overview1
Patients with intermediate-2 or high-risk MF, R/R to prior JAK inhibitor treatment, were randomized to receive intravenous imetelstat at a dose of either 9.4 mg/kg (n = 59) or 4.7 mg/kg (n = 48) every 3 weeks.
Primary endpoints:
- Spleen response rate (≥ 35% spleen volume reduction at Week 24)
- Symptom response rate (≥ 50% total symptom score reduction at Week 24)
Secondary endpoints:
- Overall survival (OS)
- Duration of response and remission
- Clinical improvement per International Working Group-Myeloproliferative Neoplasms Research and Treatment criteria
- Adverse events
- Quality of life
Exploratory endpoints:
- Pharmacokinetics and pharmacodynamics of imetelstat
Key efficacy data
Key efficacy data is summarised in Table 1.
Table 1. Imetelstat key efficacy data1
|
BM, bone marrow; CI, confidence interval; OS, overall survival; PFS, progression-free survival. |
||
|
Clinical benefit |
4.7 mg/kg imetelstat (n = 48) |
9.4 mg/kg imetelstat (n = 59) |
|---|---|---|
|
Reduction in BM fibrosis, % |
20.0 |
43.2 |
|
Symptom response at Week 24, % |
6.3 |
32.2 |
|
Spleen response at Week 24, % |
0 |
10.2 |
|
Median OS, months (95% CI) |
19.9 (17.1–33.9) |
28.1 (22.8–31.6) |
|
Median PFS, months (95% CI) |
14.8 (8.3–17.1) |
20.7 (12.0–23.2) |
|
Clinical improvement, % |
16.7 |
25.4 |
|
Transfusion independence of 12 weeks, % |
14.3 |
25.0 |
Imetelstat treatment at an active dose of 9.4 mg/kg showed clinical benefit across parameters, including BM fibrosis improvement, VAF reduction, and superior OS, when compared with the lower imetelstat dose of 4.7 mg/kg.1
Improved bone marrow fibrosis under imetelstat correlated with reduced risk of death (HR, 0.37; 95% CI, 0.14–0.98; p = 0.04).
Clinical responses were observed regardless of mutational and cytogenetic subtype, including for those with unfavorable profiles.1,2
- In the high-dose arm, spleen and symptom responses were higher in triple negative (TN) patients compared with non-TN patients, and TN patients had improved median OS (35.9 months for TN patients vs 24.6 months for non-TN patients, p = 0.05), which is surprising considering that this subgroup is associated with poor prognosis.
- Patients with high molecular risk (HMR; one or more mutations in ASXL1, EZH2, IDH1, or IDH2) had higher spleen and symptom responses, and showed a trend for improved OS when treated with the higher dose of imetelstat, compared with HMR patients treated at the lower dose.
The anti-tumor activity of imetelstat was observed at both a molecular and cytogenetic level.1,2,3
- Imetelstat treatment led to a reduction in mutational burden, with the complete elimination of some driver and non-driver gene mutations.
- In 46.2% of patients receiving 9.4 mg/kg imetelstat, there was a ≥ 20% VAF reduction in any of the mutated genes present at baseline, which was associated with increased spleen response (12.5% vs 3.0%) and symptom response (31.3% vs 24.2%), improved BM fibrosis (54.4% vs 25.0%) and longer median OS (31.6 months vs 22.8 months; HR, 0.512) compared with patients not achieving ≥ 20% VAF reduction.
- Specifically, a ≥ 25% reduction in VAF of JAK2, CALR, or MPL was seen in 42.1% of patients receiving the active imetelstat dose, compared with 5.6% of patients receiving the lower dose (p = 0.019).
- Of the 24 patients with cytogenetic abnormalities at baseline, five patients had a ≥ 50% reduction of their abnormal clones, and all had isolated 13q deletion.
Pharmacodynamic effects3
An optimal PD effect, defined as ≥ 50% reduction in telomerase activity (TA) or human telomerase reverse transcriptase (hTERT) expression, was achieved in a significantly greater proportion of patients receiving the higher dose of imetelstat, compared with the lower dose (Figure 1), and in a significantly greater proportion of patients with higher imetelstat exposure, as determined by Cmax > mean value and AUC0-24h (Figure 2).
Figure 1. Dose-dependent PD effects of imetelstat3
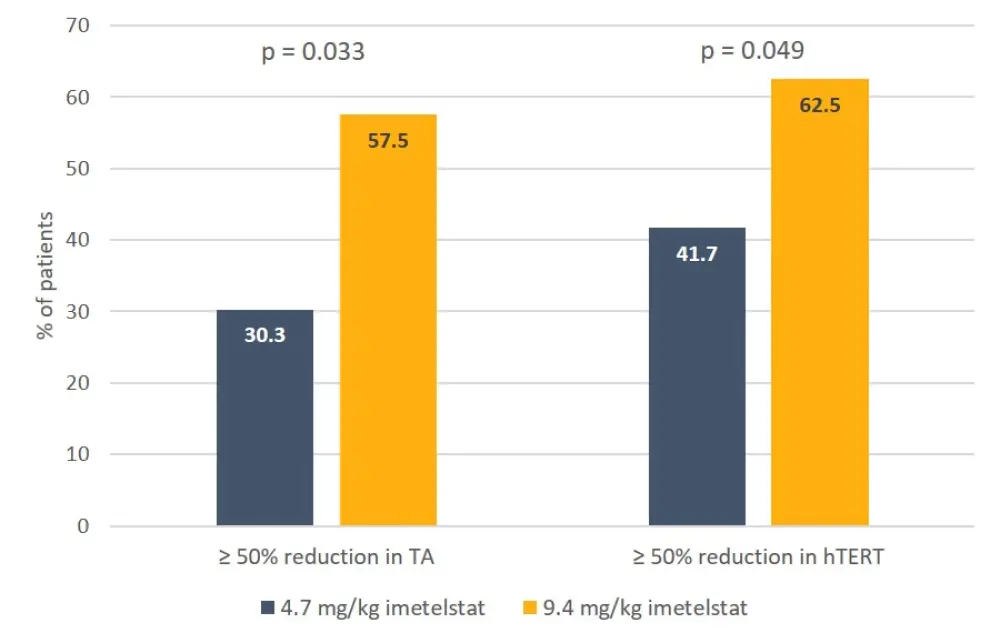
hTERT, human telomerase reverse transcriptase; TA, telomerase activity.
Figure 2. Exposure-dependent PD effects of imetelstat3
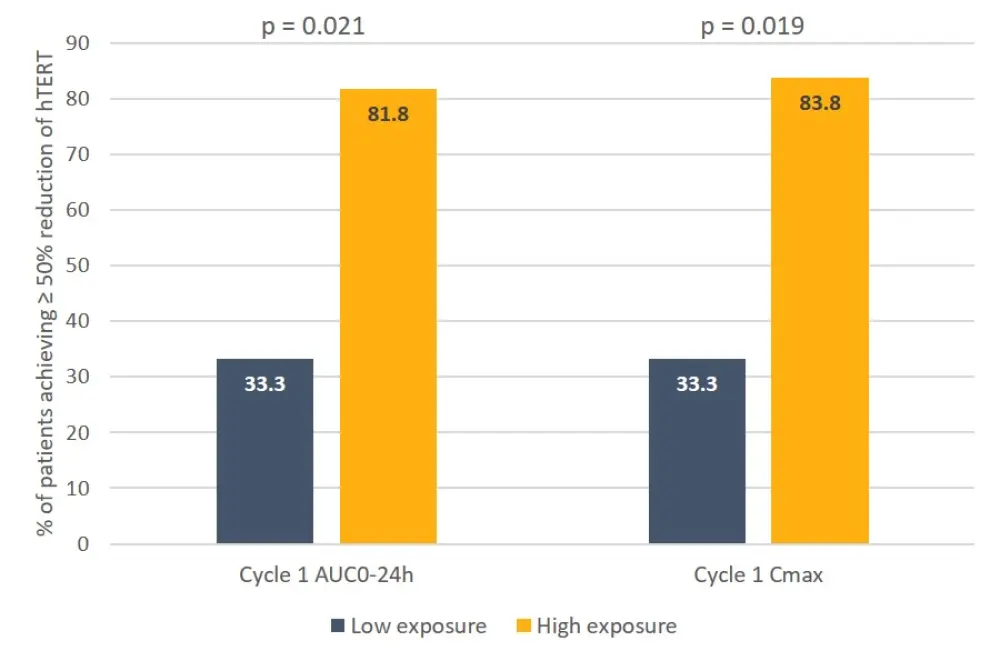
AUC0-24h, area under the plasma concentration time curve over a 24h dosing period; Cmax, maximum measured plasma concentration; hTERT, human telomerase reverse transcriptase; PD, pharmacodynamics.
- Optimal PD effect seen with the higher dose of 9.4 mg/kg imetelstat, correlated with higher rates of spleen and symptom responses and improved OS (median OS, 27.2 months vs 18.3 months for optimal PD vs those not achieving optimal PD; HR, 0.54).
- Shorter telomere length (TL; ≤ median) and higher hTERT expression levels at baseline were also associated with better clinical responses in patients receiving the higher dose of imetelstat (Table 2).
Table 2. Clinical responses according to TL and hTERT expression in patients receiving 9.4 mg/kg imetelstat3
|
hTERT, human telomerase reverse transcriptase; TL, telomere length. |
||
|
|
Patients achieving spleen response, % |
Patients achieving symptom response, % |
|---|---|---|
|
TL ≤ median |
17.3 |
37.9 |
|
TL > median |
4.2 |
25.0 |
|
hTERT ≤ median |
7.1 |
28.6 |
|
hTERT > median |
13.8 |
37.9 |
Summary
In the phase II IMbark study, patients receiving imetelstat at a triweekly dose of 9.4 mg/kg demonstrated high rates of spleen and symptom responses, and superior OS compared with patients receiving low-dose imetelstat, regardless of cytogenetic and mutational profile. The anti-tumor activity of imetelstat was observed at a cytogenetic and molecular level and disease modifying activity could be observed in patients with isolated deletion of 13q.
Pre-clinical findings suggesting a correlation between PD and anti-tumor activity were validated in this clinical cohort. Dose- and exposure-dependent reductions in TA and hTERT expression, and correlation of clinical response with shorter TL and higher hTERT expression in patients treated with imetelstat, support an on-target mechanism of action through telomerase inhibition.
Overall, the data show promise for imetelstat as a therapy for MF patients with JAK inhibitor failure and will enter phase III clinical testing (IMpactMF, NCT04576156).
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

