All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Momelotinib in MF and thrombocytopenia: MOMENTUM, SIMPLIFY-1 and SIMPLIFY-2 subgroup analysis
Do you know... What percentage of patients discontinued momelotinib treatment due to thrombocytopenia across the MOMENTUM, SIMPLIFY-1, and SIMPLIFY-2 trials?
Momelotinib, an oral inhibitor of Janus kinase 1, Janus kinase 2, and activin A type 1 receptor (ACVR1), is a U.S. Food and Drug Administration (FDA) approved therapy for the treatment of patients with anemic myelofibrosis (MF). Patients with MF often also present with thrombocytopenia, which is associated with poorer clinical outcomes.
The efficacy of momelotinib was evaluated by Kiladjian et al.1 in a post-hoc subgroup analysis of data from MOMENTUM, SIMPLIFY-1, and SIMPLIFY-2, previously reported by the MPN Hub.
Study design
Patients from the three included studies with thrombocytopenia, characterized by a platelet count <100 × 109 /L, were included. The design of this multi-trial analysis is presented in Figure 1.
Figure 1. Post hoc analysis design*
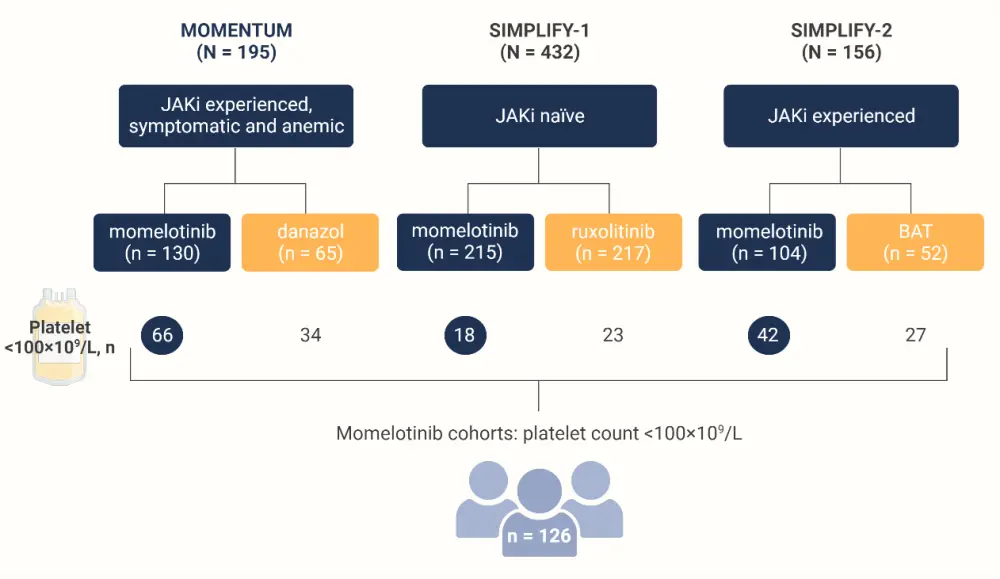
BAT, best available therapy; JAKi, Janus kinase inhibitor.
*Adapted from Kiladjian, et al.1
To assess momelotinib efficacy in the <100 × 109/L patient subgroups, data was collected at Week 24 in the following measures:
- spleen volume reduction ≥35%
- transfusion independence
- Total Symptom Score reduction ≥50%
- overall survival (OS)
Results
Response rates stratified by baseline platelet count for each trial are presented in Figure 2.
Figure 2. Response rates for SV35, transfusion independence, and TSS*
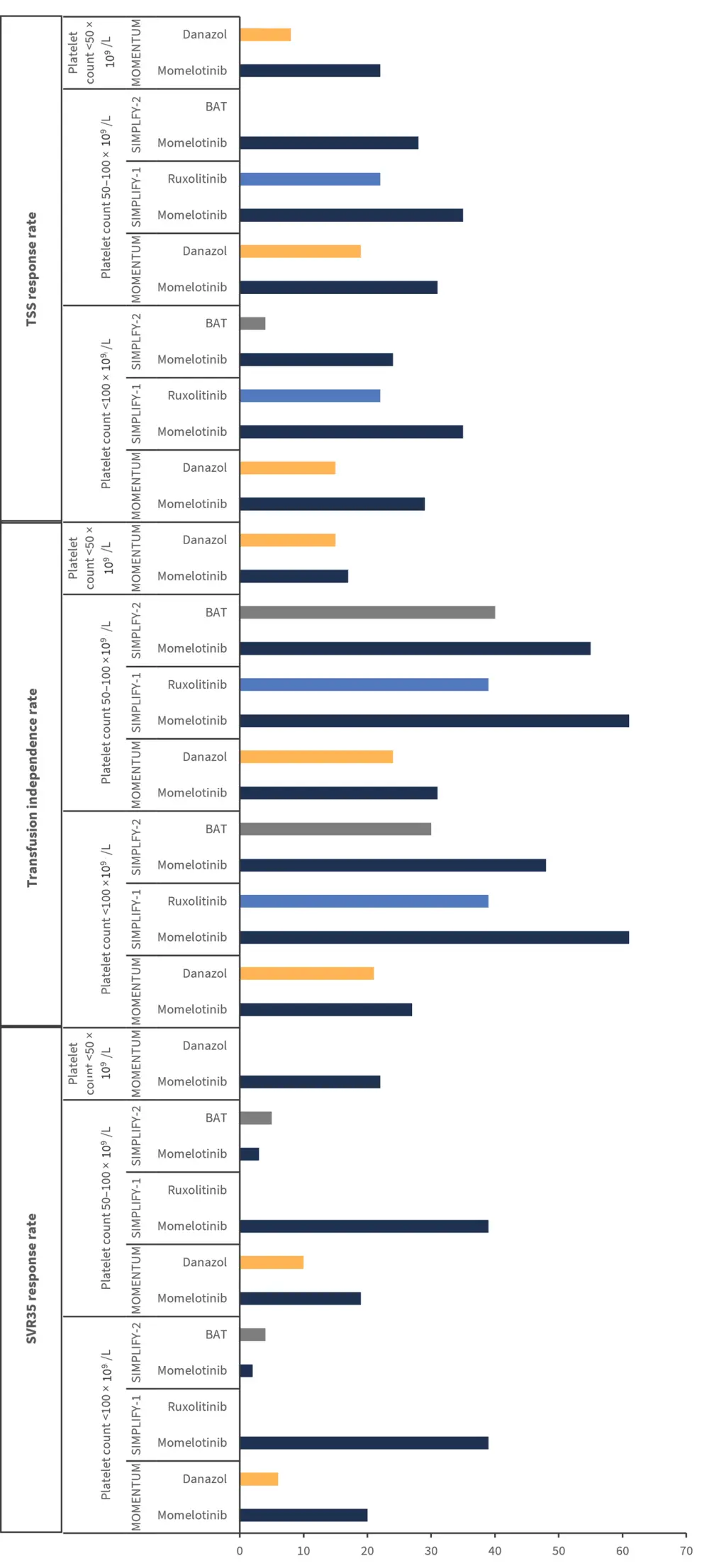
BAT, best available therapy; SVR35, spleen volume reduction ≥35%; TSS, Total Symptom Score.
*Adapted from Kiladjian, et al.1
OS data was collected at various time points and compared with the overall populations of each trial; this data is presented in Table 1.
Table 1. OS data for SIMPLIFY-1, SIMPLIFY-2, and MOMENTUM*
|
BAT, best available therapy; OS, overall survival. |
|||
|
OS, % |
<100 × 109/L |
<50 × 109/L |
Overall |
|---|---|---|---|
|
SIMPLIFY-1 |
|||
|
Momelotinib > momelotinib |
|
|
|
|
3 years |
56.7 |
— |
71.1 |
|
6 years |
37.8 |
— |
54.3 |
|
Ruxolitinib > momelotinib |
|
— |
|
|
3 years |
53.3 |
— |
71.1 |
|
6 years |
26.6 |
— |
53.3 |
|
SIMPLIFY-2 |
|||
|
Momelotinib > momelotinib |
|
— |
|
|
3 years |
59.8 |
— |
49.9 |
|
6 years |
59.8 |
— |
42.8 |
|
BAT > momelotinib |
|
— |
|
|
3 years |
65.5 |
— |
53.9 |
|
6 years |
54.6 |
— |
46.7 |
|
MOMENTUM |
|||
|
Momelotinib > momelotinib |
|
|
|
|
6 months |
86.2 |
94.4 |
88.1 |
|
12 months |
74.7 |
75.6 |
74.0 |
|
Danazol > momelotinib |
|
|
|
|
6 months |
82.1 |
59.8 |
79.9 |
|
12 months |
71.0 |
47.9 |
73.6 |
- Treatment with momelotinib consistently improved outcome measures compared with each trial comparator.
- Mean platelet counts remained consistently stable or improved following treatment with momelotinib.
- Overall, OS rates remained consistent compared with the intent to treat populations.
Safety
Grade ≥3 thrombocytopenia and hemorrhage were used as key safety indicators, alongside thrombocytopenia resulting in discontinuation or dose reduction (Table 2).
Table 2. Safety outcomes*
|
Patients, % |
MOMENTUM |
SIMPLIFY-1 |
SIMPLIFY-2 |
|||
|---|---|---|---|---|---|---|
|
|
Momelotinib |
Danazol |
Momelotinib |
Ruxolitinib |
Momelotinib |
BAT |
|
AE, adverse events; BAT, best available therapy. |
||||||
|
Grade ≥3 AEs |
|
|
|
|
|
|
|
Thrombocytopenia |
33 |
21 |
17 |
22 |
19 |
7 |
|
Hemorrhage |
6 |
0 |
6 |
4 |
7 |
0 |
|
Thrombocytopenia resulting in dose reduction or discontinuation |
12 |
5 |
17 |
— |
— |
— |
- Thrombocytopenia was the most common Grade ≥3 adverse event observed
- Discontinuation or dose reduction due to thrombocytopenia was observed in <20% of the momelotinib cohorts in MOMENTUM and SIMPLIFY-1
- Across all studies, 6% discontinued treatment due to thrombocytopenia
- There were no deaths due to thrombocytopenia
- The safety profile of the momelotinib cohorts was not significantly different from the overall safety populations
- Major adverse cardiovascular events were infrequent, and the rate of hemorrhage did not differ significantly from the overall population
Conclusion
Overall, momelotinib demonstrated a clinical benefit in patients with MF and thrombocytopenia compared with ruxolitinib, danazol, and best available therapy. The safety profile was comparable to the overall population. Notably, this post hoc analysis used trials not initially designed for platelet count subgroup analysis; however, it highlighted the potential of momelotinib as a treatment option for further investigation in patients with MF and thrombocytopenia.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

