All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Novel targeted therapies for R/R MF: PIM1 kinase inhibition
Featured:
Do you know... Which of the following statements is true of PIM1 kinase in myelofibrosis?
During the MPN Hub Steering Committee meeting, Francesco Passamonti, Università degli Studi di Milano, Milan, IT, chaired a discussion on novel targeted therapies for relapsed/refractory (R/R) myelofibrosis (MF), with a focus on PIM1 kinase inhibition. This discussion also featured Tiziano Barbui, Haifa Kathrin Al-Ali, Steffen Koschmieder, Jean-Jacques Kiladjian, Aaron Gerds, and Laura Michaelis.
Novel targeted therapies for R/R MF: PIM1 kinase inhibition
Novel targeted therapies for R/R MF: PIM1 kinase inhibition
Passamonti provided an overview of the mechanism of action of PIM1 kinase inhibitors, before presenting results from a phase I/II trial of nuvisertib, a first-in-class selective PIM1 kinase inhibitor, in patients with R/R MF.
Presentation
Janus kinase inhibitors (JAKi) are the current standard of care for patients with MF; however, therapies with novel mechanisms of action could provide alternative treatment options for patients who are either ineligible for or R/R following treatment with JAKi.1
PIM1 kinases are downstream targets of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway (Figure 1).2,3
Figure 1. Mechanism of action of PIM1 kinase inhibitors*
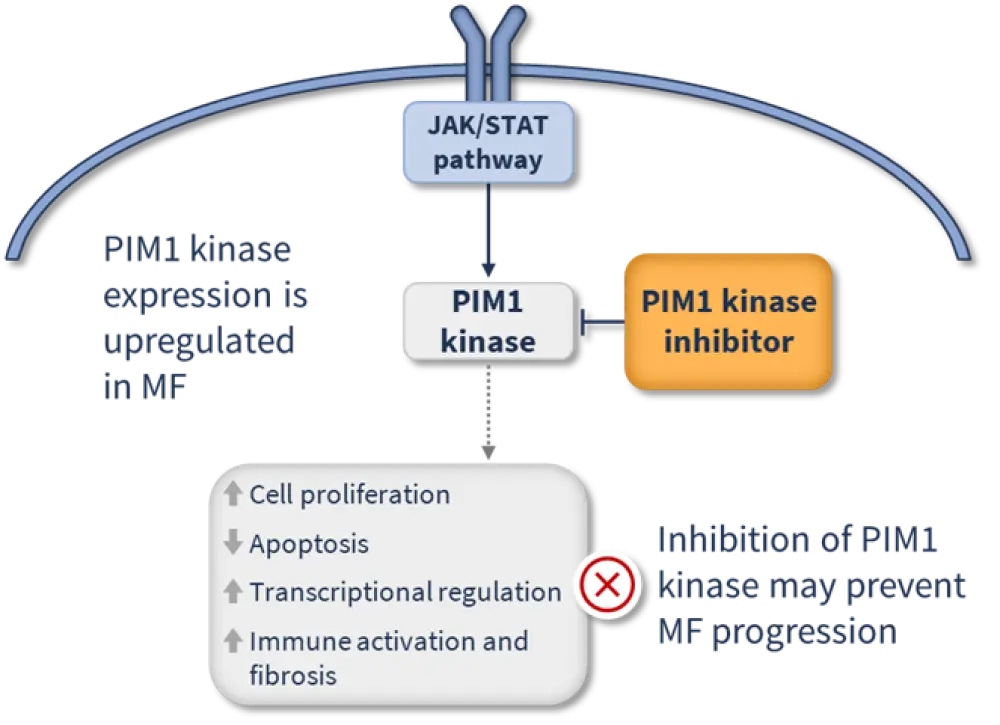
Nuvisertib, an investigational, oral, PIM1 kinase inhibitor, has been granted orphan drug and fast track designations by the U.S. Food and Drug Administration (FDA) for the indication of MF.4 Its safety and efficacy as monotherapy and in combination with JAKi therapy (ruxolitinib or momelotinib) is currently being assessed in patients with R/R MF in an ongoing, open-label, multicenter, phase Ib/II trial (NCT04176198) (Figure 2).5
Figure 2. Study design of phase Ib/II trial of nuvisertib in patients with R/R MF*
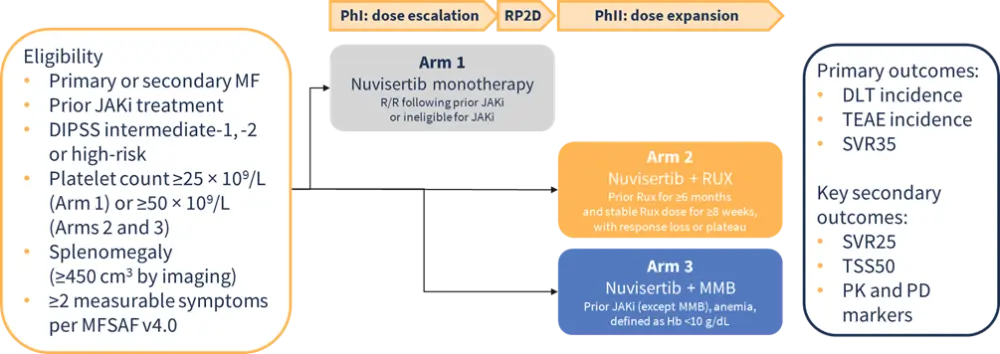
Initial results from the nuvisertib monotherapy arm (Arm 1) showed promising preliminary efficacy and a favorable safety profile in patients with R/R MF (N = 77):6
Spleen volume reduction (SVR) was observed in 67% of 18 patients, with 22.2% of patients achieving an SVR ≥25%.
Total symptom score (TSS) reduction was reported in 61.1% of 18 patients, with 44.4% of patients achieving a TSS reduction ≥50%.
Mean hemoglobin improvement ≥1.0 g/dL for ≥12 weeks without red blood cell transfusion was demonstrated in 24% of patients.
Mean platelet improvement ≥30 × 109/L for ≥28 days without platelet transfusion was observed in 26.7% of patients.
Improvement in bone marrow fibrosis ≥1 grade was observed in 42.9% of patients.
Significant modulation (p < 0.001) of pro- and anti-inflammatory cytokines (e.g. decreased EN-RAGE and MIP-1β, and increased adiponectin) was demonstrated in a longitudinal analysis.
Nuvisertib was well tolerated, with no dose-limiting toxicities reported.
Discussion
Suboptimal response in the phase Ib/II trial of nuvisertib in patients with R/R MF was defined as inadequate control of symptoms and spleen and/or requiring red blood cell transfusions; Arm 1 included patients who were R/R to or ineligible for JAKi therapy, while Arms 2 and 3 included patients with a suboptimal response to JAKi therapy.
The baseline characteristics indicate that patients in Arm 1 had advanced, high-risk MF with a significant disease burden:
Median spleen volume was 1,936 cm3.
Median spleen length was 23 cm.
Median TSS was 23.
30% of patients were transfusion-dependent.
77% were Dynamic International Prognostic Scoring System (DIPSS) intermediate-2 to high risk.
41% had a high molecular risk.
Given that both PIM1 kinase inhibitors and JAKi can be associated with thrombocytopenia, the inclusion criteria for Arms 2 and 3 require a platelet count ≥50 × 109/L.
Monitoring PIM1 kinase activity in patients receiving JAKi to identify patients with elevated kinase activity could help inform treatment selection.
Nuvisertib and JAKi have a potentially synergistic effect in patients with MF, with nuvisertib targeting PIM1 kinase downstream of the JAK/STAT pathway; therefore, nuvisertib in combination with JAKi could be particularly beneficial in patients treated with JAKi monotherapy who remain symptomatic.
There was recognition that these insights were based on a limited number of patients, and that long-term follow-up data would be necessary to understand the potential clinical impact.
This educational resource is independently supported by Sumitomo Pharma. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Jean-Jacques Kiladjian
Jean-Jacques Kiladjian Tiziano Barbui
Tiziano Barbui Laura Michaelis
Laura Michaelis Haifa Kathrin Al-Ali
Haifa Kathrin Al-Ali Steffen Koschmieder
Steffen Koschmieder Aaron Gerds
Aaron Gerds Francesco Passamonti
Francesco Passamonti
