All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Peri-transplant ruxolitinib in patients with myelofibrosis: A phase I trial
Dysregulation of the JAK-STAT pathway plays a central role in the pathogenesis of myelofibrosis (MF), a clonal myeloproliferative neoplasm, and is the main therapeutic target in its treatment. Ruxolitinib is a potent JAK 1/2 inhibitor that reduces spleen size and improves constitutional symptoms in patients with MF; however, allogeneic hematopoietic stem cell transplantation (HSCT) remains the only curative option. Generally, ruxolitinib is tapered off and stopped prior to HSCT, but early evidence suggests there may be a role for peri-transplant ruxolitinib.
Ali et al.,1 in an article published in Blood Advances, hypothesized that peri-HSCT ruxolitinib may prevent ruxolitinib discontinuation syndrome (RDS), improve engraftment, and reduce graft-versus-host disease (GvHD). The results of their pilot phase I trial (NCT02917096) evaluating the safety and efficacy of peri-HSCT ruxolitinib in patients with MF are summarized in this article.
Study design
The primary objectives of this prospective, single-center, open-label trial, were to determine the safety, maximum tolerated dose, and recommended phase II dose of ruxolitinib administered peri-HSCT in patients with MF. Secondary objectives included cumulative incidence of Grade 2–4 acute GvHD, chronic GvHD, donor cell engraftment, Grade 3–4 infection, overall survival, progression-free survival, cumulative incidence of disease relapse/progression, and non-relapse mortality.
Two dose levels (DL) of ruxolitinib were examined: 5 mg (DL1) and 10 mg (DL2). Dose-limiting toxicities (DLTs) were defined as follows:
- Regimen-related Grade 3 or 4 toxicity as per Bearman criteria
- Grade 4 neutropenia with fever
- Infection lasting ≥21 days
- Grade 4 neutropenia lasting ≥28 days (engraftment failure)
- Any other regimen-related death
- Any Grade 5 sepsis-related toxicity possibly related to ruxolitinib
Conditioning regimen, GvHD prophylaxis, and ruxolitinib treatment (Figure 1) were as follows:
- Conditioning regimen with fludarabine and melphalan:
- Fludarabine (25 mg/m2/day) infusion on Day −9 to Day −5
- Melphalan 140 mg/m2 (optional 100 mg/m2 for patients ≥70 years old) on Day −4
- GvHD prophylaxis with tacrolimus and sirolimus:
-
- Tacrolimus (0.02 mg/kg/day) continuous IV from Day −3, converting to oral dosing when tolerated
- Sirolimus 12 mg oral loading dose on Day −3, then 4 mg daily
- Ruxolitinib (5 mg or 10 mg twice daily) after conditioning from Day −3 to Day 30 (to Day −10 for patients already on ruxolitinib prior to conditioning)
- Ruxolitinib then tapered (DL1: 5 mg daily for five days then stopped; DL2: 5 mg twice daily for three days then stopped)
Figure 1. Treatment schema*
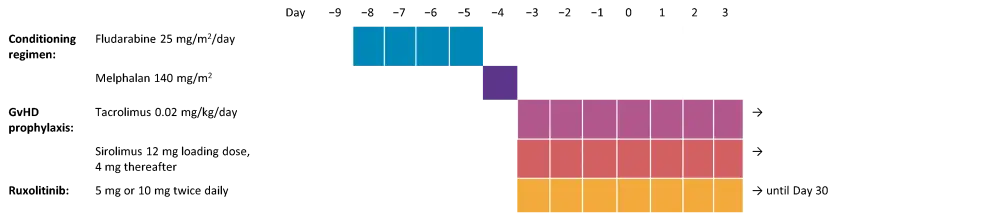
*Adapted from Ali, et al.1
Eligible patients were aged 18–75 with primary/secondary MF at intermediate-2 or high-risk disease as per the Dynamic International Prognostic Scoring System (DIPSS) and scheduled to undergo their first HSCT. Patient and HSCT characteristics are summarized in Table 1. Of note, 12 patients were being treated with ruxolitinib, of which six stopped prior to conditioning and six continued at a lower dose.
Table 1. Patient and HSCT characteristics*
|
CMV, cytomegalovirus; DIPSS, Dynamic International Prognostic Scoring System for myelofibrosis; DL, dose level; HCT CI, hematopoietic stem cell transplantation-specific comorbidity index; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplantation; MF, myelofibrosis. |
|||
|
Characteristic, % unless otherwise specified |
DL1: 5 mg |
DL2: 10 mg |
All patients |
|---|---|---|---|
|
Median age, years (range) |
53 (25–67) |
69 (55–73) |
65 (25–73) |
|
Female |
17 |
25 |
22 |
|
Male |
83 |
75 |
78 |
|
Race |
|||
|
Caucasian |
67 |
100 |
88 |
|
Asian |
17 |
0 |
6 |
|
Pacific Islander |
17 |
0 |
6 |
|
Ethnicity |
|||
|
Hispanic |
33 |
8 |
17 |
|
Non-Hispanic |
67 |
92 |
83 |
|
Baseline MF |
|||
|
Mild |
17 |
0 |
6 |
|
Moderate |
33 |
17 |
33 |
|
Severe |
17 |
83 |
61 |
|
Disease status at baseline |
|||
|
No response/stable disease |
100 |
92 |
94 |
|
Progression from hematologic |
0 |
8 |
6 |
|
Median HCT CI (range) |
1 (0–3) |
3† (1–5) |
3‡ (0–5) |
|
Performance status |
|||
|
80 |
0 |
33 |
22 |
|
90 |
67 |
59 |
61 |
|
100 |
33 |
8 |
17 |
|
MF type |
|||
|
Primary |
67 |
75 |
72 |
|
Secondary |
33 |
25 |
28 |
|
MF risk (DIPSS criteria) |
|
|
|
|
High |
17 |
25 |
22 |
|
Intermediate-2 |
83 |
75 |
78 |
|
HLA |
|||
|
7/8 |
17 |
0 |
6 |
|
8/8 |
83 |
100 |
94 |
|
Donor type |
|||
|
Sibling |
33 |
25 |
28 |
|
Unrelated |
67 |
75 |
72 |
|
Donor/Recipient CMV pre-HSCT |
|||
|
Negative/negative |
17 |
17 |
17 |
|
Negative/positive |
33 |
33 |
33 |
|
Positive/negative |
0 |
17 |
11 |
|
Positive/positive |
50 |
33 |
39 |
|
Median CD34 dose × 106/kg (range) |
6.0 (4.3–9.1) |
6.0 (3.9–8.6) |
6.0 (3.9–9.1) |
|
Median time from diagnosis to HSCT, months |
12.9 (3.0–30.8) |
27.2 (4.0–74.5) |
17.2 (3.0–74.5) |
|
Median time from diagnosis to treatment, months |
12.6 (2.7–30.5) |
26.9 (3.7–74.2) |
17.0 (2.7–74.2) |
Results
A total of 18 patients were enrolled: six in DL1 and 12 in DL2. Median follow-up was 22.6 months.
Safety
Adverse events (AEs) and DLTs are summarized in Table 2 by grade and DL. Most AEs were Grade 1 and 2. Serious AEs occurred in eight patients, all of which were considered unrelated to ruxolitinib. One patient developed DLTs at each DL: Grade 3 cardiac and gastrointestinal events and Grade 4 pulmonary event at DL1; Grade 3 kidney injury at DL2.
Table 2. Toxicity summary by organ per Bearman criteria*
|
CNS, central nervous system; DLT, dose-limiting toxicity; GI, gastrointestinal. |
||||||||||||
|
n |
DL1: 5 mg† (n = 3) |
DL1: 5 mg (n = 3) |
DL2: 10 mg (n = 12) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Grade |
Grade |
Grade |
||||||||||
|
1 |
2 |
3 |
4 |
1 |
2 |
3 |
4 |
1 |
2 |
3 |
4 |
|
|
Bladder |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
|
Cardiac |
0 |
0 |
1‡ |
0 |
0 |
0 |
0 |
0 |
4 |
0 |
0 |
0 |
|
CNS |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
1 |
0 |
0 |
|
GI |
2 |
0 |
1‡ |
0 |
2 |
0 |
0 |
0 |
9 |
0 |
0 |
0 |
|
Hepatic |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
|
Pulmonary |
1 |
0 |
0 |
1‡ |
0 |
0 |
0 |
0 |
4 |
2 |
0 |
0 |
|
Renal |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
5 |
2 |
1‡ |
0 |
|
Stomatitis |
3 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
6 |
2 |
0 |
0 |
Infectious complications included cytomegalovirus (CMV) viremia (n = 3), respiratory infections (n = 3), BK virus cystitis (n = 1), bacteremia (n = 3), and Clostridium difficile colitis (n = 4). There were no cases of RDS.
Efficacy
Engraftment:
- All patients achieved neutrophil recovery (median: DL1, 19 days; DL2 16 days).
- Platelet engraftment was established in 14/18 patients (median: DL1, 25 days; DL2 28 days).
- Steroid-refractory acute GvHD and CMV infection were contributing factors in four patients who did not achieve platelet transfusion independence.
- All patients had 100% donor chimerism at 1 year.
GvHD:
At 100 days, the cumulative incidence of acute GvHD Grade 2–4 was 17% and Grade 3–4 was 11%. Half of patients developed chronic GvHD, with a cumulative incidence of moderate/severe disease of 24% by 1 year. Individual cases of GvHD by grade/severity are listed in Table 3.
Table 3. Summary of cases of GvHD*
|
*Adapted from Ali et al.1 |
||
|
|
DL1: 5 mg |
DL2: 10 mg |
|---|---|---|
|
Maximum grade of acute GvHD, n |
||
|
None |
3 |
6 |
|
1 |
2 |
4 |
|
2 |
0 |
1 |
|
3 |
1 |
1 |
|
4 |
0 |
0 |
|
Overall severity of chronic GvHD, n |
||
|
None |
1 |
8 |
|
Mild |
3 |
1 |
|
Moderate |
1 |
2 |
|
Severe |
1 |
1 |
Survival outcomes:
- 1-year overall survival: 77% (95% CI, 50–91)
- 1-year progression-free survival: 71% (95% CI, 44–87)
- Estimated 1-year GvHD-free/relapse-free survival: 52% (95% CI, 26–73)
- Cumulative incidence of relapse at 1 year: 6% (95% CI, 1–40)
- 1-year non-relapse mortality: 23% (95% CI, 10–54)
- Causes of death: cardiac arrest (n = 1), GvHD (n = 2), and respiratory failure related to severe mucositis (n = 1).
Immune reconstitution, plasma cytokines, and GvHD biomarkers:
- A trend towards faster recovery of CD3+ T cells, CD8+ cells, and CD27+ memory B cells was seen in patients on DL2 at Days 21, 21, and 35, respectively, compared with DL1.
- Plasma cytokine levels (IL-2, IFN-ɣ, TNF-α) were significantly lower at DL2 compared with DL1 (p ≤ 0.05).
- No significant difference in GvHD biomarkers was seen at either dosage level.
Conclusion
In this high-risk cohort of patients with MF, the addition of ruxolitinib peri-HSCT was found to be safe and well tolerated. Rates of GvHD were favorable compared with previous research assessing allogeneic-HSCT with a fludarabine/melphalan conditioning regimen and tacrolimus/sirolimus GvHD prophylaxis, and survival rates were promising. The addition of peri-HSCT ruxolitinib did not increase infection rates, and the authors suggested that careful, planned tapering may have contributed to the absence of RDS. Therefore, ruxolitinib dosing at 10 mg twice a day was recommended for further larger phase II randomized trials to further assess the benefits of ruxolitinib in the peri-transplant setting.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

