All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
PERSIST trials: Pooled analysis of pacritinib in patients with MF and severe thrombocytopenia
Severe thrombocytopenia, defined as platelet counts of <50 × 109/L, is a recognized adverse prognostic factor in patients with myelofibrosis (MF). This patient subset often experiences anemia, greater risk of bleeding, worse symptom burden, higher risk of leukemic transformation, and shorter survival in contrast to patients with higher platelet counts.1 As a result, patients are often excluded from clinical trials and lack access to effective treatment options.
Ruxolitinib and fedratinib have been approved for the treatment of MF; however, they have been associated with treatment-related thrombocytopenia and have not been evaluated in patients with severe thrombocytopenia. Pacritinib is a novel Janus kinase 2 (JAK2)/interleukin-1 receptor-associated kinase 1 inhibitor currently under evaluation for patients with MF and thrombocytopenia. It was granted accelerated approval by the U.S. Food and Drug Administration (FDA) for the treatment of patients with MF and severe thrombocytopenia, based on the results of two phase III trials, PERSIST-1 (NCT01773187) and PERSIST-2 (NCT02055781), which represent the largest data set published in this patient population.
Here, we summarize the findings of a recent study by Verstovsek et al.1 published in Haematologica, further exploring the efficacy and safety data in patients with severe thrombocytopenia enrolled in the PERSIST studies compared with a cohort treated with the best available therapy (BAT).
Methods
The PERSIST-1 and PERSIST-2 study design and methodology have been described previously here. Key elements are summarized below.
PERSIST-1
- Enrolled patients regardless of platelet count.
- PERSIST-1 randomized patients 2:1 to receive 400 mg pacritinib daily or BAT.
PERSIST-2
- Enrolled patients with platelet counts ≤100 × 109/L.
- Prior use of JAK inhibitors and ruxolitinib was permitted.
- Randomized patients 1:1:1 to receive 400 mg pacritinib daily, 200 mg pacritinib twice daily, or BAT.
- Patients randomized to receive BAT were allowed to crossover to pacritinib at 24 weeks or at disease progression. Safety and efficacy data were censored at the time of crossover.
Both studies included adult patients with primary or secondary MF and severe thrombocytopenia; this population comprised 16% of patients in PERSIST-1 and 45% of patients in PERSIST-2. BAT included any available physician-selected treatment, including ‘Watch and wait’. Fedratinib was not available as BAT in either study.
Results
Figure 1 below illustrates the population for the pooled analysis. Table 1 summarizes selected baseline patient and disease characteristics in patients treated with pacritinib or BAT; there were no statistically significant differences between two treatment groups.
Figure 1. Study population*
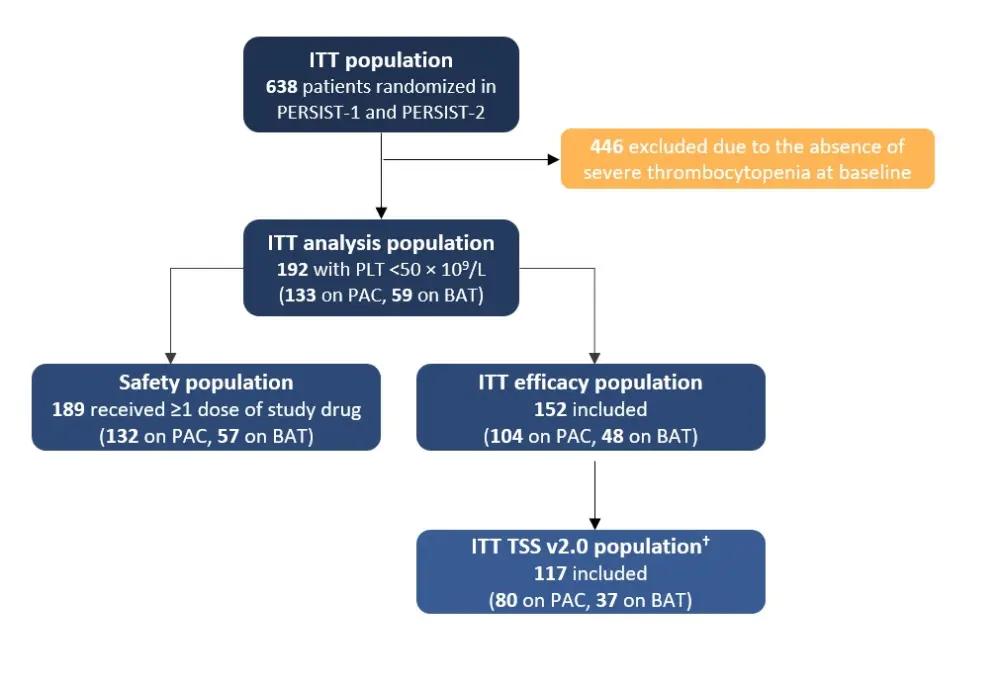
BAT, best available therapy; ITT, intention-to-treat; PAC, pacritinib; PLT, platelets; TSS, Total Symptom Score.
*Adapted from Verstovsek et al.1
†As the Total Symptom Score (TSS) instrument administered during PERSIST-1 was changed from v1.0 to v2.0 part-way through the study, only patients who had completed v2.0 at baseline were included in the intention-to-treat (ITT) TSS analysis.
Table 1. Patient baseline characteristics*
|
BAT, best available therapy; IQR; interquartile range; JAK2, Janus kinase 2; MF, myelofibrosis; PET, post-essential thrombocythemia; PPV, post-polycythemia vera; TSS, Total Symptom Score. |
||
|
Characteristic |
Pacritinib arm |
BAT arm |
|---|---|---|
|
Age, median (range) |
69 (50–91) |
69 (50–84) |
|
MF diagnosis, % |
||
|
Primary MF |
74 |
67 |
|
PPV-MF |
15 |
14 |
|
PET-MF |
11 |
19 |
|
Time since MF diagnosis (years), median (IQR) |
2.0 (0–27) |
2.6 (0–14) |
|
Prior JAK2 inhibitor, % |
33 |
37 |
|
Platelet count (109/L), |
29 |
25 |
|
Hemoglobin <10 g/dL, % |
64 |
63 |
|
Spleen volume at baseline (cm3) †, |
2,566 |
2,466 |
|
Modified TSS score at baseline†, |
17 |
17 |
- Grade 3 bone marrow fibrosis was observed in 49% of patients.
- The most common BAT therapies were ‘Watch and wait’ (37%), ruxolitinib (30%), hydroxyurea (28%), and prednisone (12%).
- Duration of the study drug exposure was similar for pacritinib and BAT (median 5.5 and 5.2 months, respectively).
- Short treatment durations were partly due to truncation at the time of the clinical hold; 46% of patients were still on pacritinib at the time of the clinical hold.
- The median total daily dose of pacritinib remained 400 mg at Weeks 12 and 24, whereas patients in PERSIST-2 who received ruxolitinib as BAT were prescribed a median post-titration dose of 10 mg twice daily and were on this treatment for a median duration of 3.45 months.
Efficacy analysis
Efficacy outcomes were evaluated at Week 24 and included the percentage of patients achieving ≥35% spleen volume reduction (SVR), achieving ≥50% reduction in modified TSS, and reporting symptoms as being ‘much’ or ‘very much’ improved, based on the Patient Global Impression of Change (PGIC) scale (Figure 2). Given that the PERSIST-2 study was terminated prematurely due to a clinical hold, intention-to-treat (ITT) efficacy analyses included all randomized patients in PERSIST-1 and the 71% in PERSIST-2 who were randomized at least 22 weeks prior to the hold.
Figure 2. Efficacy of pacritinib versus BAT based on 24-week response rates*
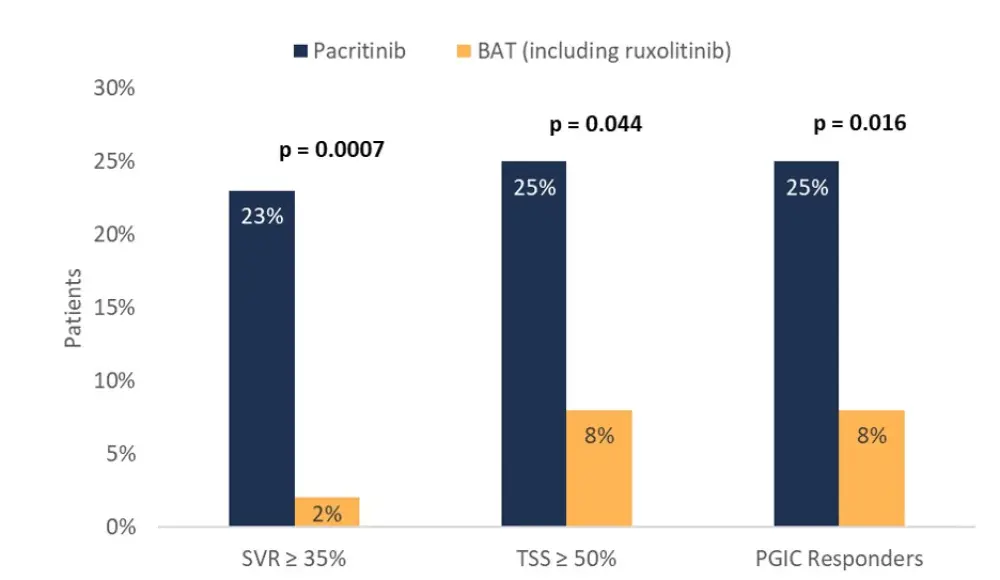
BAT, best available therapy; PGIC, Patient Global Impression of Change; SVR, spleen volume reduction; TSS, Total Symptom Score.
*Adapted from Verstovsek et al.1
- Higher response rates were reported in patients treated with 200 mg pacritinib twice daily versus those on 400 mg daily (29.0% vs 20.5%).
- The median percentage change in spleen volume was greater for pacritinib-treated patients than for BAT-treated patients (−29.4% vs −1.3%; p < 0.0001), and the percentage of patients who experienced any improvement (>0%) in SVR was higher in the pacritinib group compared with the BAT group (56.7% vs 33.3%; p = 0.0089).
- The median reduction in modified TSS score was greater with pacritinib than with BAT (−30.3% vs 0%; p = 0.0036).
- Response rates for TSS were similar between the two pacritinib doses.
- The percentage of patients who experienced any improvement (>0%) in TSS was higher in the pacritinib group compared to the BAT group (53% vs 32%; p = 0.049).
- Subgroup analyses revealed that pacritinib was associated with higher response rates regardless of whether patients had received prior treatment with JAK2 inhibitors or whether they had primary versus secondary MF.
Safety analysis
The treatment-emergent adverse events (TEAEs) in patients with severe thrombocytopenia were consistent with the PERSIST study results and were generally Grade 1 or 2 in severity (Table 2).
Table 2. Most common TEAEs (≥10% all grade or ≥3% Grade 3 or 4)*
|
BAT, best available therapy; LRT, lower respiratory tract; TEAEs, treatment-emergent adverse events; URT, upper respiratory tract. |
||||
|
TEAE, % |
Pacritinib (n = 132) |
BAT (n = 57) |
||
|---|---|---|---|---|
|
All Grade |
Grade 3–4 |
All Grade |
Grade 3–4 |
|
|
Nonhematologic |
||||
|
Diarrhea |
60.6 |
5.3 |
15.8 |
1.8 |
|
Nausea |
30.3 |
1.5 |
12.3 |
1.8 |
|
Vomiting |
26.5 |
0.8 |
7.0 |
1.8 |
|
Epistaxis |
15.9 |
6.8 |
26.3 |
1.8 |
|
Peripheral edema |
15.9 |
1.5 |
22.8 |
0 |
|
Fatigue |
14.4 |
4.5 |
10.5 |
5.3 |
|
Dizziness |
13.6 |
1.5 |
3.5 |
0 |
|
Pyrexia |
12.9 |
0 |
10.5 |
0 |
|
Constipation |
12.9 |
0.8 |
7.0 |
0 |
|
Abdominal pain |
12.1 |
1.5 |
17.5 |
1.8 |
|
Dyspnea |
10.6 |
1.5 |
8.8 |
3.5 |
|
Pneumonia |
10.6 |
7.6 |
3.5 |
3.5 |
|
Decreased appetite |
10.6 |
2.3 |
7.0 |
0 |
|
URT infection |
9.1 |
0 |
10.5 |
1.8 |
|
Contusion |
9.8 |
0 |
10.5 |
0 |
|
Cough |
7.6 |
0.8 |
12.3 |
0 |
|
Cardiac failure |
3.8 |
3.8 |
5.3 |
3.5 |
|
Atrial fibrillation |
1.5 |
0.8 |
7.0 |
3.5 |
|
General health deterioration |
3.0 |
3.0 |
0 |
0 |
|
LRT infection |
3.0 |
0 |
3.5 |
3.5 |
|
Sepsis |
3.0 |
1.5 |
5.3 |
3.5 |
|
Abdominal pain, upper |
5.3 |
0.8 |
5.3 |
3.5 |
|
Hematologic |
||||
|
Thrombocytopenia |
34.8 |
34.8 |
21.1 |
21.1 |
|
Anemia |
31.8 |
31.8 |
21.1 |
19.3 |
|
Neutropenia |
6.1 |
5.3 |
7.0 |
7.0 |
|
Leukopenia |
5.3 |
3.8 |
3.5 |
3.5 |
- Gastrointestinal events rarely led to dose reduction (3.0% for diarrhea, 1.5% for nausea) or discontinuation (3.8% for diarrhea).
- Hematologic TEAEs were generally Grade 3 or 4 given the degree of cytopenias present at baseline, but these rarely led to dose reduction (4.5% and 2.3% for thrombocytopenia and anemia, respectively) or discontinuation (3.8% for both).
- While thrombocytopenia was observed more often on pacritinib, there was no excess of hemorrhagic events (pacritinib vs BAT: Grade ≥1, 51.5% vs 59.6%; Grade 3–4, 13.6% vs 10.5%; fatal, 2% vs 0%).
- Among patients remaining on study, hemoglobin and platelet counts were stable through Week 24.
- High-grade and fatal cardiac events were observed at similar rates on pacritinib and BAT (Grade 3–4, 9.1% vs 14%; fatal, 3% vs 1.8%).
- Survival was similar between pacritinib- and BAT-treated patients: Hazard ratio, 1.01 (95% confidence interval [CI], 0.57–1.80).
Conclusion
Overall, these results from a retrospective analysis suggest that pacritinib is a promising treatment for patients with MF who lack reliable and effective therapies due to severe thrombocytopenia. Patients treated with pacritinib had improved SVR and symptom response compared to those in the BAT group. Pacritinib was well-tolerated at full doses and although rates of bleeding were higher in patients with severe thrombocytopenia, this was irrelevant of whether they were treated with pacritinib or BAT.
About the PAC203 study
The phase II PAC203 study (NCT03165734) compared the efficacy and safety of different pacritinib dosing schedules in patients with advanced and heavily pre-treated MF, including those with severe thrombocytopenia, who no longer benefitted or were intolerant to JAK inhibitors. Results showed that the SVR response rate for patients with severe thrombocytopenia was 17%, similar to that noted in this study. Variations could be due to the enrollment of patients with extended prior ruxolitinib exposure in the PAC203 study. You can find more information on PAC203, here.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

