All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Predicting transition in prefibrotic myelofibrosis
With the 2016 revision to the World Health Organization classification of myeloproliferative neoplasms (MPN), patients with prefibrotic primary myelofibrosis (pre-PMF) are recognized as a separate clinical entity. However, these patients often present with heterogeneous symptoms and their clinical course usually shows progression, with transition to overt PMF, acute myeloid leukemia (AML), or even to death. In a paper published in Blood Cancer Journal, Alessandra Carobbio and colleagues, including MPN Hub Steering Committee members Alessandro M. Vannucchi and Tiziano Barbui, set out a new multistate model of progression and survival for patients with pre-PMF.1
Study design
Retrospective data obtained at four Italian centers from 382 patients with pre-PMF were used to create the model, and the baseline patient characteristics are shown in Table 1. Median follow-up duration was 6.89 years (range, 0.08−32.6). Median age was 57.6 years and 45% of patients were > 60 years old. Lactate dehydrogenase levels were > 1.5 times the normal range in 40% of patients and 15% had palpable splenomegaly > 5 cm.
Table 1. Baseline patient characteristics1
|
LDH, lactate dehydrogenase; WBC, white blood cell. |
|||
|
Feature |
N = 382 |
5th–95th percentiles |
|
|---|---|---|---|
|
Male % |
51 |
|
|
|
Median age, years (range) |
57.6 (15.6−91.9) |
|
|
|
Median hemoglobin, g/dL |
13.5 |
9.3−15.9 |
|
|
Median hematocrit, g/dL |
41.1 |
30.6−49.0 |
|
|
Median platelet count, x 109/L |
700 |
130−1,481 |
|
|
Median WBC count, x 109/L |
10.0 |
5.0−24.1 |
|
|
Fibrosis grade, % |
0 |
42 |
|
|
1 |
58 |
|
|
|
Palpable splenomegaly, % |
46 |
|
|
|
LDH > normal range, % |
73 |
|
|
|
Abnormal cytogenetics, % |
15 |
|
|
Eligibility was decided according to the revised 2016 World Health Organization criteria along with the presence of specific genotypes and mutations, e.g., JAK2V617F, MPLW515L/K, and CALR. High molecular risk (HMR) mutations were recorded in 132 patients, including ASXL1, EZH2, SRSF2, and IDH1/2. Patients were categorized as HMR if they had one (28%) or more than one (8%) mutation and the proportions present are shown in Table 2.
Table 2. Mutations present in the study poulation1
|
HMR, high molecular risk. |
||
|
Mutation |
% |
Allele burden, median (range) |
|---|---|---|
|
Driver mutations |
||
|
JAK2V617F |
65 |
36.8 (0.3−100) |
|
CALR type 1 |
22 |
50 (9−63.7) |
|
CALR type 2 |
18 |
49 (7−94) |
|
MPLW515L/K |
5 |
|
|
Triple negative |
8 |
|
|
Non driver mutations |
||
|
HMR |
28 |
|
|
HMR 2 |
8 |
|
Key findings
Multistate model of transition
Survival was analyzed using the 4-state model shown in Figure 1, and the risk-factors associated with the transition between states were investigated. The probability of progression from pre-PMF is represented by transitions (T)1−3. The risk of transition to overt PMF (T1) was 15.2%, while the risk of evolution to AML (T2) was 4.7% and the risk of death (T3) was 17.5%.
Figure 1. Multistate model of transition to overt PMF, AML, or death1
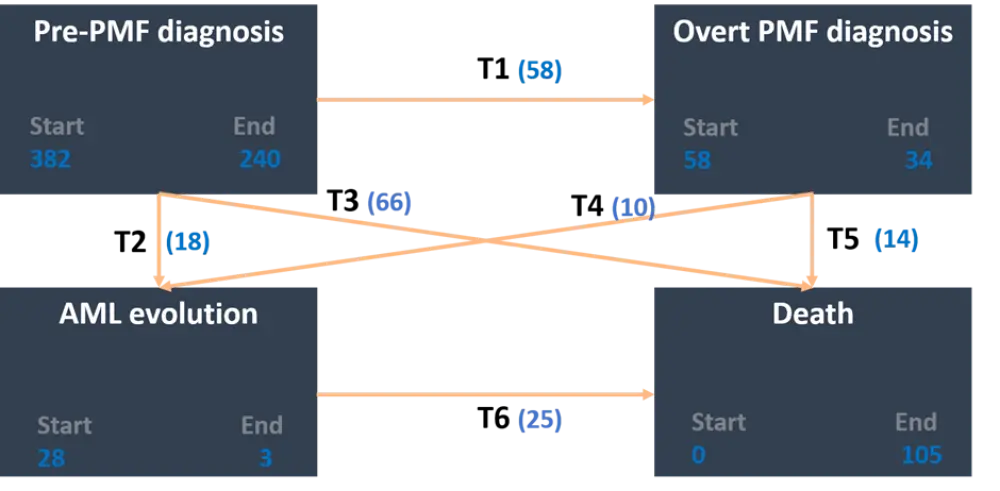
AML, acute myeloid leukemia; PMF, primary myelofibrosis; T, transition.
Numbers in blue represent number of patients.
Transition probabilities over time
The likelihood for patients to transition from overt PMF to AML was highest in the first 5 years (7%) but then decreased to < 1%. The change from overt PMF (T5) and from AML (T6) to death increased more rapidly than the other transitions. The probability for patients to progress from pre-PMF increased over time in an almost linear fashion. Around 70% of patients remained in pre-PMF after 10 years falling to 30% after 20 years. While 30% to 60% of patients died after 10 and 20 years, respectively. The probability of being alive with a diagnosis of overt PMF or AML increased over time and remained stable at 20–22% collectively after 5–10 years.
Prognostic factors of transition
The variables that were significantly associated with the three transitions from pre-PMF are shown in Table 3. For T1 (pre-PMF to overt PMF), anemia and Grade 1 fibrosis were significantly associated with an increased risk of transition. Age > 65 years, white blood cells > 15 × 109/L, and lactate dehydrogenase > 1.5 were significant risk factors for the transition from pre-PMF to AML (T2). Being > 65 years old and with leukocytosis > 15 × 109/L were also significantly associated with T3, the transition from pre-PMF to death. The presence of one HMR mutation was significantly associated with increased risk of T1 and T3, whereas having two or more HMRs was associated with T2 (Table 3).
Table 3. Risk factors associated with increased probability of transition1
|
AML, acute myeloid leukemia; CI, confidence interval; HMR, high molecular risk; HR, hazard ratio; LDH, lactate dehydrogenase; PMF, primary myelofibrosis; T, transition; ULN, upper limit of normal; WBC, white blood cells. HMR: presence of one of ASXL1, EZH2, SRSF2, and IDH1/2. HMR 2: presence of 2+ of ASXL1, EZH2, SRSF2, and IDH1/2. Bold font indicates statistical significance. |
||||||
|
Covariate |
T1 |
T2 |
T3 |
|||
|---|---|---|---|---|---|---|
|
|
HR (95% CI) |
p value |
HR (95% CI) |
p value |
HR (95% CI) |
p value |
|
Clinical |
||||||
|
Age > 65 years |
0.56 (0.25–1.30) |
0.176 |
10.3 (2.33–45.6) |
0.002 |
6.53 (2.38–17.9) |
<0.0001 |
|
Anemia |
2.18 (1.01–4.72) |
0.047 |
1.34 (0.31–5.92) |
0.695 |
2.30 (0.88–5.37) |
0.153 |
|
WBC > 15 × 109/L |
0.91 (0.29–2.84) |
0.870 |
4.80 (1.22–18.9) |
0.025 |
3.49 (1.35–9.01) |
0.010 |
|
Fibrosis grade 1 |
3.20 (1.08–9.41) |
0.035 |
0.34 (0.09–1.29) |
0.112 |
2.96 (0.82–10.7) |
0.100 |
|
LDH > 1.5 × ULN |
1.58 (0.76–3.30) |
0.221 |
8.13 (1.46–45.2) |
0.017 |
0.84 (0.36–1.98) |
0.687 |
|
Abnormal cytogenetics |
1.60 (0.64–4.00) |
0.310 |
6.92 (1.61–29.8) |
0.009 |
1.84 (0.71–4.76) |
0.209 |
|
Mutational |
||||||
|
HMR |
3.15 (1.08–9.21) |
0.036 |
— |
— |
4.62 (2.09–10.2) |
<0.0001 |
|
HMR 2 |
— |
— |
6.62 (1.11–39.3) |
0.038 |
— |
— |
Using the Akaike’s information criterion, the authors compared a model with clinical criteria only to a model combining clinical criteria with mutational analysis. While predictions for transition from pre-PMF to AML and death were similar between both models, the combined clinical and mutational model was better at predicting transition from pre-PMF to overt PMF.
Conclusion
With this multistate model the risk for transition from pre-PMF to overt PMF was calculated as 15.3%, which is in line with previous findings. However, a more granular view on the transition from pre-PMF to AML is provided here, which may occur either directly (64% of cases) or indirectly through an intermediary state of overt PMF (36% of cases).
A limitation of this model is that information on the number of HMR mutations was only available for a third of the study population, which limited the power of the analysis and prevented a prognostic score being created.
Nonetheless, the mutational model was shown to be more accurate for predicting the transition from pre-PMF to overt PMF. The predictions made by this multistate model should help guide clinical decision making and treatment planning.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

