All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Presence of abnormal karyotypes in essential thrombocythemia impacts fibrotic/leukemic progression and overall survival
The incidence of cytogenetic abnormalities in essential thrombocythemia (ET) is low at <10%, and the prognostic relevance of these abnormalities remains poorly understood. The traditional survival assessment is based on the International Prognostic Score for ET (IPSET-survival) and Mutation-Enhanced International Prognostic Scoring System for ET (MIPSS-ET) which include U2AF1, SF3B1, and TP53 mutations, while polycythemia vera (PV) and primary myelofibrosis (PMF) prognostication take into account abnormal karyotypes.1
Naseema Gangat and colleagues conducted a retrospective study1 to outline the prevalence and spectrum of cytogenetic abnormalities, identify the clinical and molecular correlations of abnormal karyotypes compared to normal karyotypes, and determine the implications of abnormalities for disease evolution and survival in context of existing prognostic models. The results were published in the Blood Cancer Journal,1 and we are pleased to summarize the findings below.
Study design
A total of 809 patients were recruited from the clinical myeloproliferative neoplasm database at the Mayo Clinic, Rochester, US:
- Median age was 59 years, and 65% of patients were female.
- JAK2-mutated cases with hemoglobin levels >16 g/dL in women and >16.5 g/dL in men were excluded (n = 22) to minimize inclusion of patients with masked PV.
- Similarly, anemic patients with Hb levels <11 g/dL in women (n = 39) and <12.5 g/dL in men (n = 40) were also excluded to avoid inclusion of patients with prefibrotic myelofibrosis (MF).
Based on the available cytogenetic assessment, patients were analyzed for normal and abnormal karyotype including the number/type of abnormal karyotypes. Patient characteristics and clinical outcomes were then compared between those with normal and abnormal karyotypes.
Results
The results of the assessment of cytogenetic karyotypes are shown in Figure 1.
Figure 1. Distribution of A genetic karyotypes among total patients (N = 809), and B abnormalities present in patients with abnormal karyotype excluding Y chromosome (n = 16)*
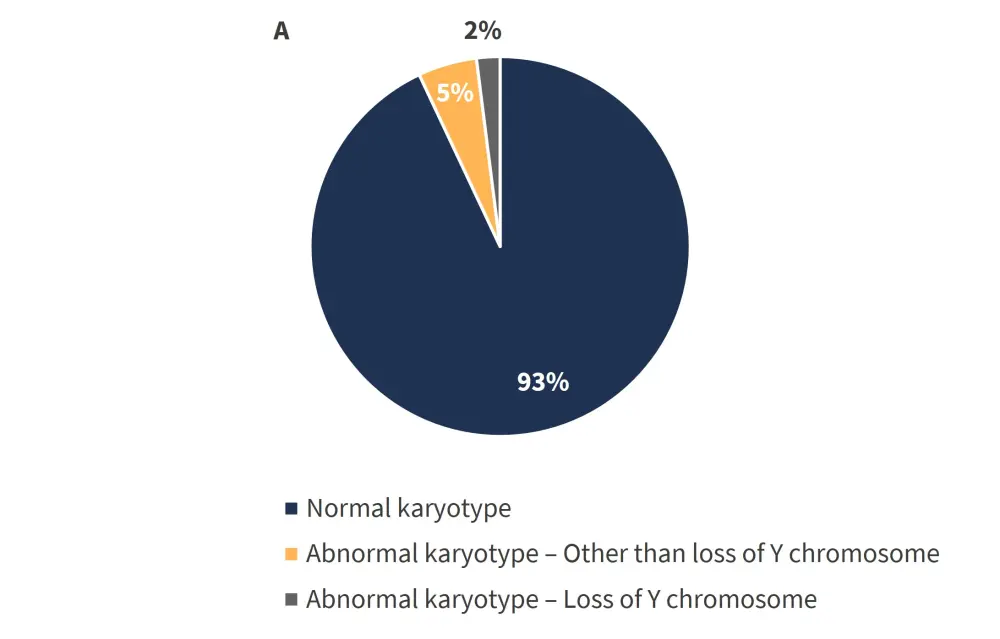
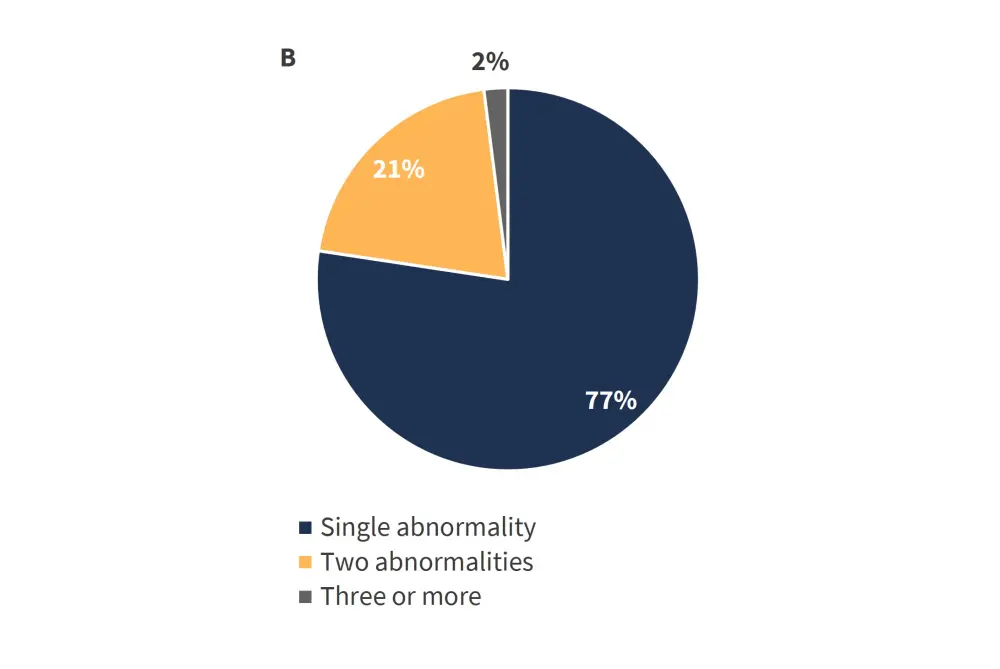
*Adapted from Gangat et al.1
The most frequent sole genetic abnormalities identified are shown in Figure 2.
Figure 2. Most common sole genetic abnormalities*
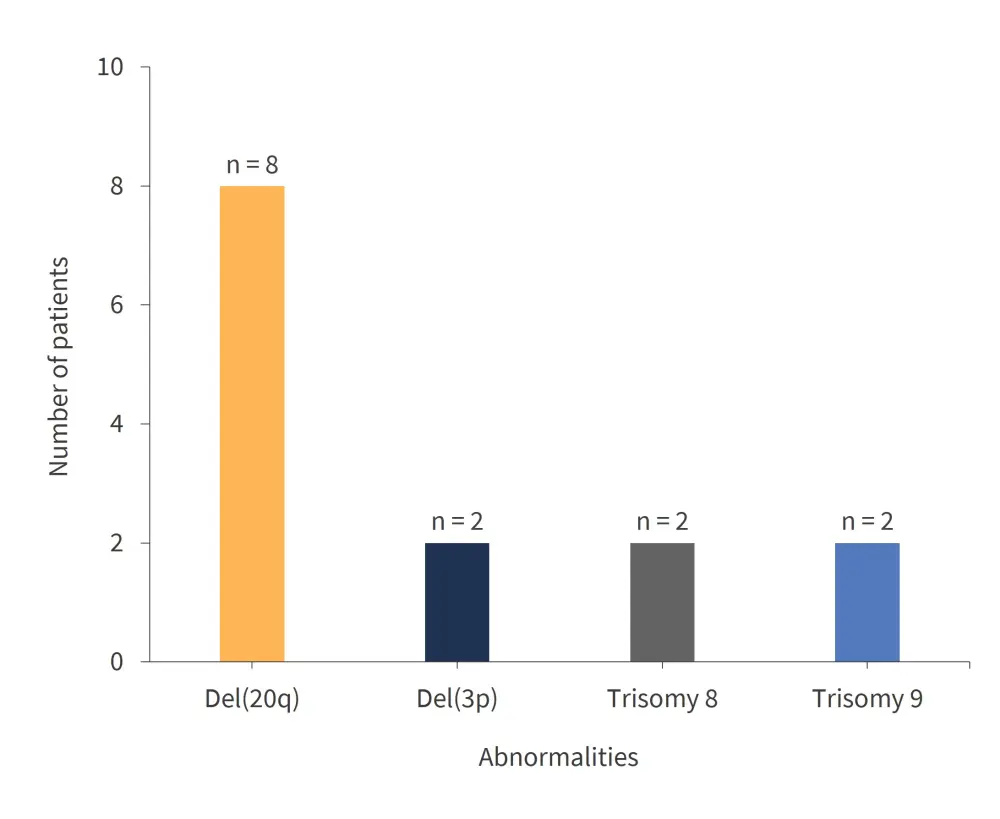
*Adapted from Gangat et al.1
Abnormal vs normal karyotype
Table 1 provides a comparative summary of statistically significant clinical and laboratory characteristics between patients with normal (n = 754) and abnormal karyotype (n = 55).
Table 1. Clinical and laboratory characteristics of patients with normal vs abnormal karyotype*
|
NGS, next-generation sequencing. |
|||||
|
Characteristic |
Normal karyotype |
Abnormal karyotype |
|||
|---|---|---|---|---|---|
|
Loss of Y chromosome |
p value† |
Excluding loss of Y chromosome |
p value† |
||
|
Age, years, median (range) |
58 |
72 |
0.001 |
64 |
0.04 |
|
>60 years, % |
44 |
81 |
0.003 |
46 |
0.24 |
|
Hemoglobin, g/dL, median (range) |
13.8 |
14.9 |
0.001 |
13.6 |
0.96 |
|
Leukocytes, 3.5–9.6 × 109/L, median (range) |
8.4 |
9.4 |
0.11 |
9.4 |
0.03 |
|
Arterial thrombosis at/prior to diagnosis, % |
13 |
25 |
0.15 |
26 |
0.02 |
|
NGS, evaluable |
n = 211 |
n = 4 |
n = 9 |
||
|
ASXL1, % |
4 |
50 |
<0.0001 |
0 |
0.55 |
The presence of del(20q) alone depicted a male majority at 75% vs 33%, and a higher incidence of prior arterial events at 38% vs 13% which was accounted for by male gender.
A total of 596 patients from the cohort were annotated for driver mutations and their association to each karyotype (Figure 3).
Figure 3. Driver mutations for each karyotype*
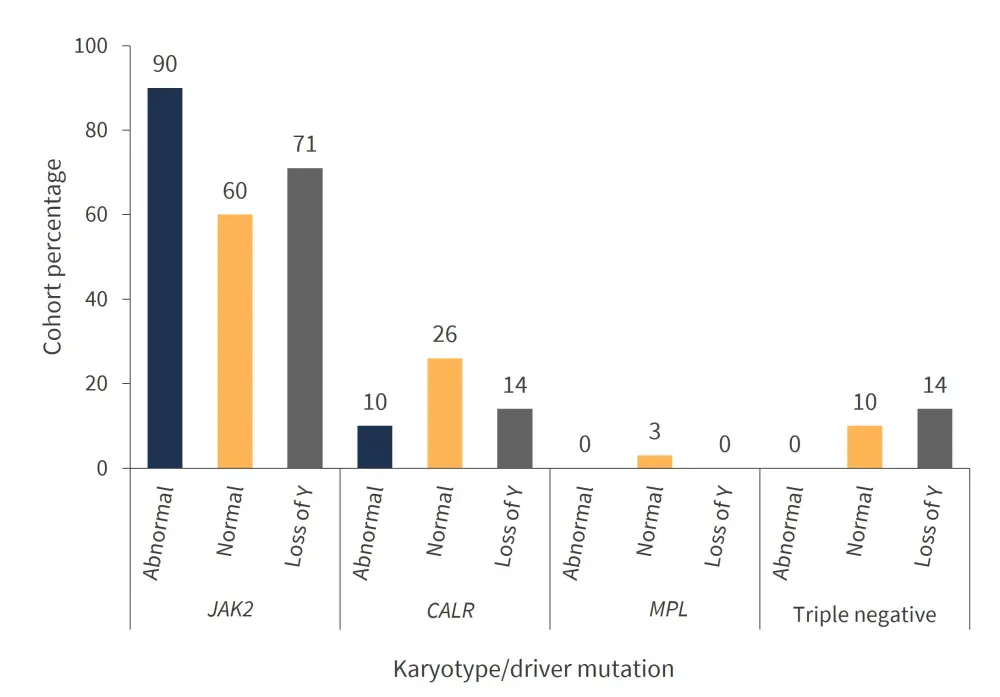
*Adapted from Gangat et al.1
Clinical outcomes
The median follow-up was 9.6 years (range, 0.01–41.2), by which time, 95 (12%) patients had undergone fibrotic transformation. Of these, 89 (12%) had the normal karyotype, 5 (13%) abnormal, and 1 (6%) had loss of Y chromosome (p = 0.77). Several predictors of fibrotic progression were identified by univariate analysis: age >60 years (p = 0.02); male gender (p = 0.04); and SF3B1/U2AF1 mutations (p = 0.001). However, no abnormal karyotype (p = 0.74) or loss of Y chromosome (p = 0.95) were identified. Leukemic transformation rates were similar across normal, abnormal, and loss of Y chromosome with 5%, 3%, and 0% frequencies, respectively (p = 0.71).
The median survival time for the abnormal and loss of Y chromosome was 12 and 10 years, respectively, compared to 21 years for the normal karyotype (p < 0.0001). Moreover, overall survival of patients with loss of Y chromosome in >75% vs <25% metaphases were significantly reduced at 5 years vs 15 years (p = 0.04); despite similar age, leukocyte count, and previous thrombosis history. The survival difference between patients with loss of Y chromosome in >75% metaphases against abnormal karyotype was fully accounted for by median age at 72 and 64 years, respectively.
Univariate analysis identified several risk factors for overall survival:
- Abnormal karyotype (p = 0.007)
- Loss of Y chromosome (p = 0.003)
- Age > 60 years (p < 0.0001)
- Leukocytosis, >11 × 109/L (p < 0.0001)
- Male gender (p = 0.0003)
- History of thrombosis (p = 0.001)
Multivariable analysis identified similar factors:
- Abnormal karyotype other than loss of Y (HR, 2.0; p = 0.001)
- Age >60 years (HR, 4.5; p < 0.0001)
- Leukocytosis, >11 × 109/L (HR, 1.5; p = 0.002)
- Male gender (HR, 1.4; p = 0.005)
The prognostic impact on overall survival of the abnormal karyotype other than the loss of Y chromosome remained significant in the presence of SF3B1, SRSF2, U2AF1, and TP53 mutations (p = 0.04).
Conclusion
The presence of the abnormal karyotype was shown to reduce overall survival, as well as magnifying the risk of fibrotic and leukemic progression. Moreover, the abnormal and loss of Y karyotypes were found to be associated with inferior survival. The study,1 the first of its kind, as well as including the largest cohort of patients so far, shows value in making use of cytogenetic studies as part of a standard diagnostic workup for patients with ET. Furthermore, for the first time, it demonstrates the impact of an abnormal karyotype other than the Y chromosome on overall survival. It however remains to be determined if the DNA-damaging effects of cytoreductive therapies in conjunction with abnormal karyotypes and/or additive somatic mutations may be enhanced.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



