All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Rusfertide interruption leads to loss of therapeutic benefit in patients with polycythemia vera
The optimal management of patients with polycythemia vera (PV) poses a challenge; uncontrolled hematocrit levels <45% are associated with thrombotic events and greater risk of mortality.1
Standard therapies include phlebotomy or cytoreductive therapy, which may not be effective and tolerable in some patients. Rusfertide, a hepcidin mimetic, is a first-in-class, novel agent currently being developed for the treatment of patients with PV and uncontrolled erythrocytosis with standard therapy.1
During the Society of Hematologic Oncology (SOHO) 2022 Annual Meeting, Naveen Pemmaraju presented updated results from the REVIVE study (NCT04057040), a phase II trial evaluating the use of rusfertide as an add-on to therapeutic phlebotomy with or without cytoreductive therapy in patients with phlebotomy-dependent PV.1 We summarize the results presented below.
Study design
The patient eligibility criteria included:
- phlebotomy-dependent PV; and
- ≥3 phlebotomies in 6 months with or without concurrent cytoreductive therapy.
Hematocrit levels were maintained at <45% with phlebotomy prior to rusfertide treatment. Subcutaneous doses of 10–120 mg rusfertide given weekly were added to prior standard therapy. There were three parts to the study design:
- Part 1: Dose finding and efficacy evaluation (28 weeks)
- Part 2: Randomized withdrawal phase (up to 12 weeks)
- Part 3: Open-label extension phase (up to 36 months)
The primary endpoint of the study was the proportion of patients achieving a response. Patient response was defined as having achieved the absence of “phlebotomy eligibility”, a hematocrit level >45% that was ≥3% higher than baseline, or a hematocrit level >48% during the efficacy evaluation phase beginning Week 17 through Week 20. The clinical goal was to maintain hematocrit levels <45%.
The full study design and patient characteristics can be found here.
Results
A total of 57 patients who completed the first 28 weeks of dosing (Part 1) were evaluable for phlebotomy requirement. Study results showed that there was a significant decrease with rusfertide in the phlebotomy requirement in patients treated with phlebotomy only (n = 29; p < 0.0001) and patients treated with both phlebotomy and cytoreductive therapy (n = 28; p < 0.0001) (Figure 1). The mean number of phlebotomies in total during the 28 weeks before rusfertide add-on was 4.81 (median, 4; range, 2–10) and after adding rusfertide, the mean number of phlebotomies reduced to 0.30 (median, 0; range, 0–2).
Figure 1. Mean rate of phlebotomies before and during 28 weeks of dosing for patients treated with A phlebotomy only or B phlebotomy and cytoreductive therapy*
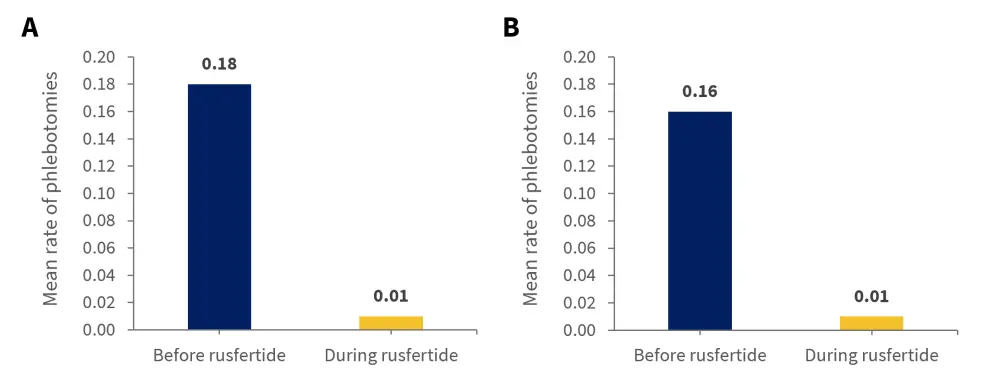
*Adapted from Pemmaraju, et al.1
Interruption and reinitiation
During a brief clinical hold in the study of ~5 weeks as a result of preclinical mouse models showing four cases of cancer, including non-melanoma skin cancer, interruption of rusfertide resulted in a loss of effect in phlebotomy rate, yet the restarting of rusfertide restored the therapeutic benefit. Rusfertide interruption resulted in an increase in hematocrit levels and decrease in ferritin levels over time. A similar pattern of reduction in red blood cell counts was also observed; however, there was no significant change in platelet or leukocyte counts. This clinical effect was recorded in 48 patients. These patients were able to return to rusfertide add-on treatment 2–3 months after the interruption and reinitiation.
Safety
The most common treatment-emergent adverse events (TEAEs) were Grade 1 and 2, with 80% of patients recording an injection site reaction; none of these reactions led to treatment discontinuation. No Grade 3 TEAEs related to rusfertide were recorded and there was no Grade 4 or 5 TEAEs recorded across the entire cohort. Table 1 shows the any grade TEAEs recorded in ≥10% of patients.
Table 1. TEAEs of any grade in ≥10% of patients*
|
TEAE, treatment-emergent adverse event. |
|
|
TEAE, n (%) |
n = 70 |
|---|---|
|
Injection site reaction |
56 (80.0) |
|
Fatigue |
19 (27.1) |
|
Pruritis |
17 (24.3) |
|
Arthralgia |
16 (22.9) |
|
Headache |
15 (21.4) |
|
Dizziness |
13 (18.6) |
|
Nausea |
12 (17.1) |
|
Anemia |
11 (15.7) |
|
Hyperhidrosis |
9 (12.9) |
A total of two patients withdrew from the study due to TEAEs (popliteal aneurysm and pulmonary embolism). Five patients developed secondary malignancies during the study; a total of six skin cancers, three of which were squamous cell carcinoma, two were basal cell carcinoma, and one was malignant melanoma. However, all these patients had previous exposure to ruxolitinib and/or hydroxyurea therapy. Moreover, of these five patients, one developed acute myeloid leukemia but had recorded prior exposure to radioactive iodine treatment.
Conclusion
Results from the study showed that rusfertide provides effective hematocrit control and has comparable efficacy in patients only treated with phlebotomy as well as those treated with phlebotomy and cytoreductive therapy. The treatment was also well tolerated with patients being treated for up to 18 months and remained mostly phlebotomy free. Data beyond this time period however is unavailable and should therefore be closely monitored. Rusfertide treatment was suspended briefly due to a clinical hold and the loss in therapeutic benefit as a result was subsequently regained once treatment had been restarted. The VERIFY trial (NCT05210790), a multinational randomized phase III study has subsequently begun enrollment.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



