All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Ruxolitinib adherence in patients with MF or PV
The efficacy of ruxolitinib (RUX) is dependent on continuous administration in patients with myeloproliferative neoplasms.1 Discontinuation is associated with splenomegaly re-expansion and disease symptoms in myelofibrosis (MF) and increased hematocrit levels and disease symptoms in polycythemia vera (PV).1 Overall, poor adherence increases the risk of treatment failure.
Recently, Palandri et al.1 published results from the RAMP study (NCT06078319) in the Annals of Hematology, evaluating the incidence of low adherence to RUX and associated factors, including psychological stress, in patients with MF and PV.
Study design1
- This was a prospective multicenter study that enrolled patients diagnosed with MF or PV between January 1985 and December 2021.
- Patients completed the validated Adherence to Refills and Medications Scale (ARMS) and Distress Thermometer and Problem List (DTPL).
- The ARMS is made up of 12 questions to patients that require answers via a 4-point Likert scale, with scores of 1 equaling never and 4 equaling always.
- Eight items investigate intentional or non-intentional non-adherence, while four items investigate logistical or financial drug supply influences.
- For the DTPL analysis, patients were asked to indicate their level of distress on a scale of 0 (no emotional distress/stress) to 10 (maximum distress/stress) on an analog scale.
Key findings1
- A total of 189 patients completed at least one ARMS/DPTL test.
- 141 patients had MF, and 48 patients had PV.
Observations at Week 0
- 49.7% of patients reported low adherence in Week 0, and of these patients:
- 48.9% indicated issues with the RUX supply process; and
- 46.8% reported unintentional non-adherence.
- Globally, low adherence was comparable between patients with MF and PV (48.2% vs 54.2, respectively).
- The percentage of patients with high psychological distress was similar in those with MF (39.7%) and PV (41.7%).
Adherence and distress over time
- Among patients who completed all tests (n = 138):
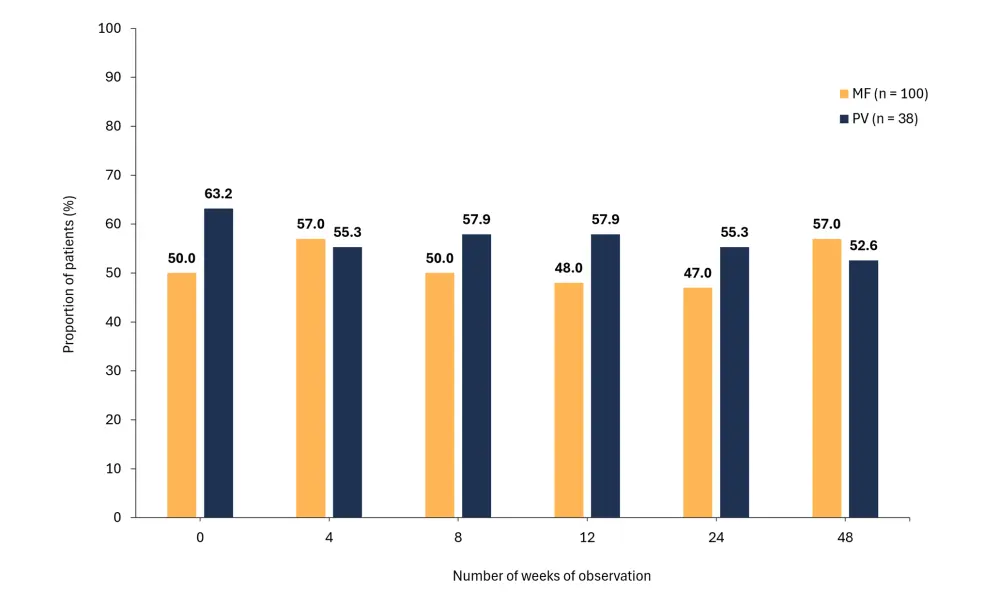
- Adherence and distress varied over the 48-week observation period but on the whole remained consistent (Figure 1).
- 36.9% consistently reported low adherence through to Week 48.
- Stable low adherence was more frequent in patients with PV vs MF (52.6% vs 31%, respectively; p = 0.02)
- Cases of unintentional non-take decreased from 47.8% (Week 0) to 26% (Week 48).
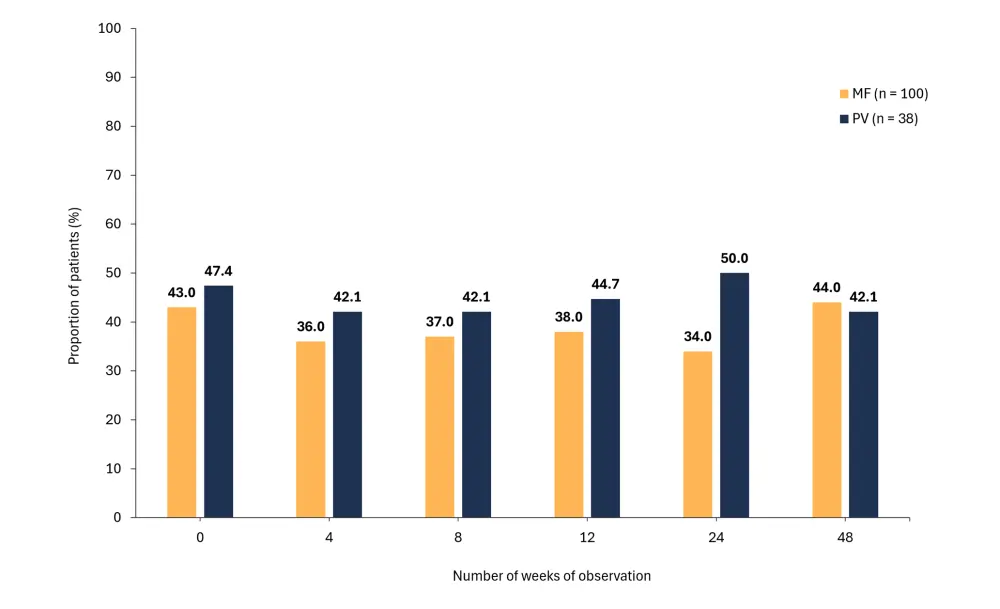
Figure 1. Percentage of patients with MF or PV who completed all tests and declared A low adherence to RUX and B a high level of distress*
A
B
MF, myelofibrosis; PV, polycythemia vera.
*Adapted from Palandri, et al.1
- 22.5% of patients reported always having high distress; this was comparable between MF and PV (p = 0.83).
- Overall, 61 patients with MF consistently reported high adherence from Week 0 to Week 24.
- The probability of achieving a spleen response was higher among patients with MF who always reported low distress (n = 69; p = 0.04)
|
Key learnings |
|---|
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

