All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Ruxolitinib is a Janus kinase (JAK) 1/2 inhibitor that is licensed for the treatment of patients with myelofibrosis (MF) and is effective in controlling MF-related splenomegaly and inducing hematological responses. The approval was based on results from the COMFORT-I and -II (NCT00952289 and NCT00934544) phase III trials.1 In the original phase I/II study of ruxolitinib (NCT00509899), 92% of patients discontinued treatment, mainly due to associated toxicities or lack of response/disease progression. Of these patients, 11% developed severe withdrawal symptoms during ruxolitinib discontinuation.2
Ruxolitinib discontinuation syndrome (RDS) occurs within 21 days of treatment stop and is characterized by an acute relapse of disease symptoms, accelerated splenomegaly, worsening of cytopenias, and occasional hemodynamic decompensation, including acute respiratory distress syndrome and shock.2 RDS occurs regardless of the ruxolitinib dose, tapering, and steroid usage.1
In order to investigate RDS in a real-world setting, a large cohort (N = 700) of patients with MF, treated with ruxolitinib across 21 mostly Italian hematology centers, were assessed. These results were presented by Francesca Palandri as oral presentation during the 25th European Hematology Association Annual Congress.1
Aims1
- Outline the modalities of ruxolitinib discontinuation
- Describe the incidence, timing, and severity of RDS
- Investigate the outcome and risk factors associated with RDS
Methods1
- A survey was conducted in all 21 participating centers to obtain comprehensive information regarding the timing and modalities of ruxolitinib discontinuation and clinical/laboratory data during treatment, at the time of discontinuation, and after discontinuation
- Data cutoff: May 1, 2020
- RDS was defined as follows:
- Mild: If symptoms did not require any medical interventions
- Moderate: If symptoms required medical interventions, such as administering steroids, oral analgesics, or restarting ruxolitinib
- Severe: If symptoms required intravenous medications, hospital admission, splenectomy, or caused the delay of hematopoietic stem cell transplantation (HSCT)
- Fatal: If death was attributable to RDS
Results1
- After a median follow-up time of 36.1 months from the start of ruxolitinib, 251 patients (35.9%) survived ≥ 30 days after discontinuation and were evaluable for analysis
- Patient characteristics at the time of ruxolitinib discontinuation can be seen in Table 1
- Most patients were > 70 years of age and presented with anemia and a large splenomegaly
- The ruxolitinib dose was < 10 mg twice daily in over 50% of patients
Table 1. Patient characteristics at the time of ruxolitinib discontinuation1
|
BLCM, below left costal margin; Hb, hemoglobin; PLT, platelets; WBC, white blood cells |
|
|
Characteristic |
(n = 251) |
|---|---|
|
Male, % |
60.6 |
|
Primary myelofibrosis, % |
39.8 |
|
> 70 years of age, % |
53.0 |
|
Hb < 10 g/dL, % |
68.5 |
|
Median PLT count, × 109/L (range) |
103 (3–976) |
|
PLT count < 100 × 109/L, % |
53.8 |
|
WBC count > 25 × 109, % |
27.5 |
|
Spleen > 10 cm BLCM, % |
45.8 |
|
Total symptom score > 20, % |
27.5 |
|
Ruxolitinib dose, mg twice daily, % |
|
|
20 |
15.1 |
|
15 |
15.6 |
|
10 |
23.1 |
|
5 |
46.2 |
Management of ruxolitinib discontinuation1,2
- Ruxolitinib was immediately stopped in 64.5% of patients; in the remaining 35.5% of patients, the ruxolitinib dose was gradually decreased by 5 or 10 mg/day at variable intervals (range, 3–30 days)
- The median duration of tapering was 14 days
- In 38.2% of patients that received ruxolitinib tapering, it was carried out in combination with prednisone (12.5 mg/day) ± hydroxyurea (1 g/day) administration
- In 13 centers, tapering was performed in < 20% of cases
- Only four centers regularly performed tapering, and tapering use was consistent within individual centers
RDS incidence1
- 13.5% of patients developed RDS, mostly mild (Figure 1)
- The median time to all grades of RDS was 7 days (range, 2–21 days)
Figure 1. RDS incidence1
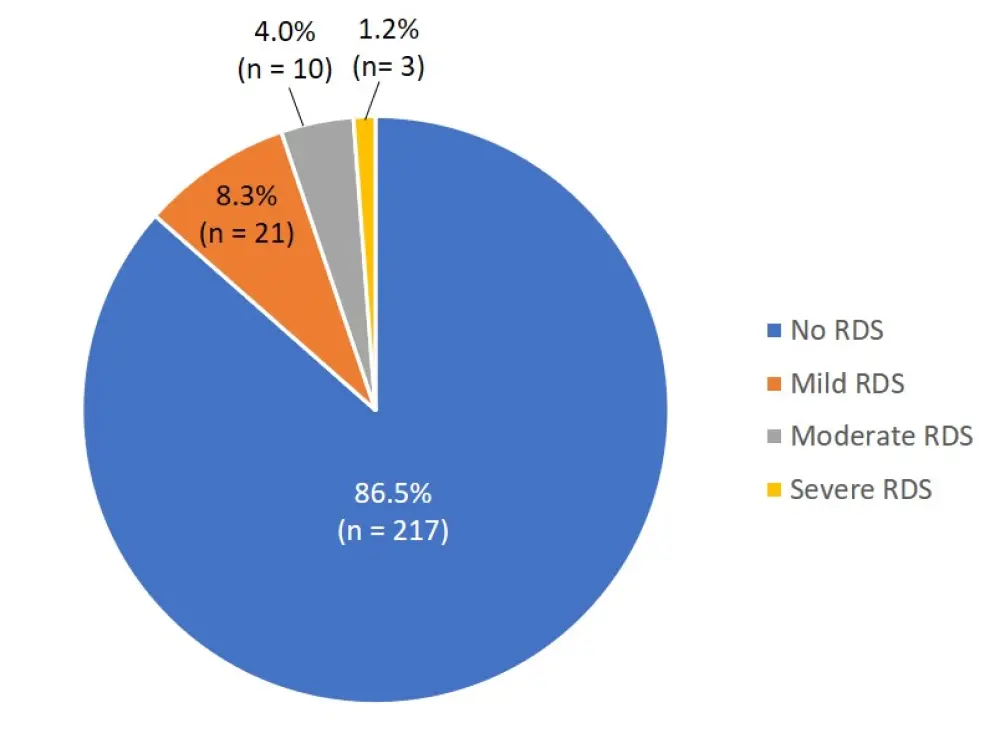
RDS, ruxolitinib discontinuation syndrome
- The most common symptom in 21 patients with mild RDS was an increase of the spleen (62% of patients), followed by fatigue, itching, bone pain, and abdominal discomfort (29% of patients). Night sweats, fever, and weight loss were seen in 9% of patients
- The median time to mild RDS was 10 days (range, 3–21 days)
- An increased spleen was also seen in ten patients (70%) with moderate RDS, while two patients had night sweats, fever, and weight loss. One patient had extreme fatigue
- The median time to moderate RDS was 8.5 days (range, 3–20 days)
- Severe RDS occurred only in three patients (1.2%), and no fatal cases were reported
Outcomes1
- Mild RDS required no intervention by definition, however, 38% of patients received various therapies, such as hypomethylating agents, chemotherapy, splenectomy, or allo-HSCT, and 15% received JAK2-inhibitors (Figure 2A)
- Of the patients with moderate RDS, eight received corticosteroids and two received investigational non-JAK2 inhibitors. Three patents subsequently received ruxolitinib re-challenge after an average time of 2.6 years (Figure 2B)
Figure 2. Outcomes and treatments for patients with mild and moderate RDS1
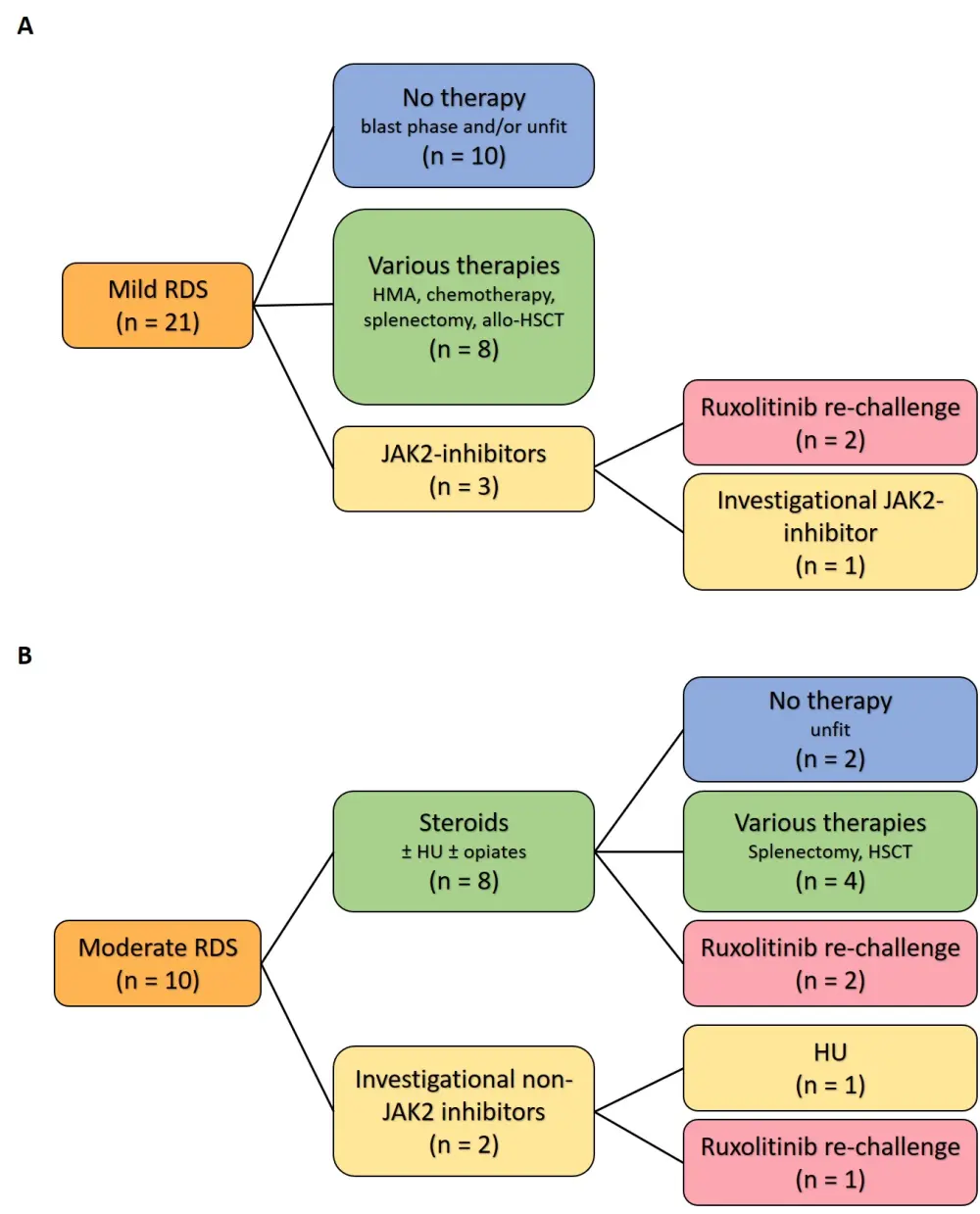
allo-HSCT, allogeneic hematopoietic stem cell transplantation; HMA, hypomethylating agent; HU, hydroxyurea; JAK2, Janus kinase 2; RDS, ruxolitinib discontinuation syndrome
- Severe RDS occurred in three patients (Table 2) within 3 days of discontinuation and caused various symptoms
- All patients rapidly recovered after ruxolitinib re-challenge at 10 mg twice daily
Table 2. Treatment and outcome of patients with severe RDS1
|
BLCM, below left costal margin; CMV, cytomegalovirus; Hb, hemoglobin; HU, hydroxyurea; MF, myelofibrosis; MOF, multiple organ failure; PLT, platelets; PMF, primary myelofibrosis; PPV, post-polycythemia vera; RDS, ruxolitinib discontinuation syndrome; TSS, total symptom score; WBC, white blood cells |
|||
|
|
Case 1 |
Case 2 |
Case 3 |
|---|---|---|---|
|
Age, years |
48 |
65 |
74 |
|
Gender |
Male |
Male |
Female |
|
MF type |
PPV |
PMF |
PMF |
|
Ruxolitinib dose, mg twice daily |
20 |
15, reduced to 10 |
10, reduced to 5 |
|
Duration of ruxolitinib, months |
6.2 |
39.6 |
3 |
|
Reason for discontinuation |
Thrombocytopenia |
Lack of response |
Thrombocytopenia |
|
Patient characteristics at ruxolitinib discontinuation |
|
|
|
|
Spleen size, cm BLCM |
15 |
14 |
14 |
|
TSS |
0/100 |
70 |
2 |
|
WBC count, × 109/L |
84.3 |
9.1 |
41.6 |
|
Hb, g/dL |
11 |
8.2 |
7.4 |
|
PLT count, × 109/L |
55 |
58 |
45 |
|
Ruxolitinib discontinuation |
Rapid decrease to 5 mg, then immediate stop, without steroids |
24 days of tapering with steroids |
Immediate stop |
|
Time to RDS, days |
1 |
3 |
2 |
|
RDS symptoms |
Spleen rupture, systemic symptoms, hyperleukocytosis |
Fever, dyspnea, confusion, dizziness |
Acute respiratory distress syndrome |
|
Treatment |
Splenectomy, no response to steroids and HU, rapid resolution after ruxolitinib 10 mg twice daily |
Steroids, rapid clinical improvement after ruxolitinib 10 mg twice daily |
Steroids, resolution of clinical symptoms after ruxolitinib 10 mg twice daily |
|
Follow up period, months |
48 |
19.2 |
3.6 |
|
Last contact |
Dead: MOF after cholecystectomy |
Alive: receiving ruxolitinib 15 mg twice daily |
Dead: CMV lung infection |
Risk Factors for RDS1
- Table 3 shows that the factors at the time of ruxolitinib stop that were significantly associated with increased risk of RDS (univariate analysis) were platelet count < 100 × 109/L (HR, 2.83; p = 0.01), ruxolitinib dose ≤ 10 mg twice daily (HR, 4.95; p = 0.01), and spleen size ≥ 10 cm (HR, 2.15; p = 0.03)
Table 3. Risk factors for RDS (univariate analysis)1
|
BLCM, below left costal margin; CI, confidence interval; Hb, hemoglobin; HR, hazard ratio; MF, myelofibrosis; PPV, post-polycythemia vera; PET, post–essential thrombocythemia; PLT, platelets; TSS, total symptom score Bold font indicates statistical significance. |
||
|
Risk factor |
HR (95% CI) |
p value |
|---|---|---|
|
Age ≥ 70 years |
0.79 (0.41–1.56) |
0.51 |
|
Male gender |
0.92 (0.46–1.83) |
0.83 |
|
PPV/PET MF |
1.05 (0.53–2.07) |
0.89 |
|
Hb < 10 g/dL |
1.73 (0.75–3.99) |
0.19 |
|
PLT < 100 × 109/L |
2.83 (1.28–6.23) |
0.01 |
|
Tapering |
1.89 (0.96–3.71) |
0.06 |
|
Ruxolitinib dose ≤ 10 mg twice daily |
4.95 (1.51–16.20) |
0.01 |
|
Spleen ≥ 10 cm BLCM |
2.15 (1.06–4.37) |
0.03 |
|
TSS ≥ 20 |
0.73 (0.31–1.69) |
0.46 |
- Multivariable analysis revealed that two factors at the time of ruxolitinib discontinuation were significantly associated with a higher probability of developing RDS:
- Platelet count < 100 × 109/L (HR, 2.98; 95% CI, 1.29–6.90; p = 0.01)
- Spleen size ≥ 10 cm (HR, 2.03; 95% CI, 1.01–4.17; p = 0.04)
Conclusion
Severe RDS is rare and occurs in approximately 1% of patients. Diagnosis of RDS is crucial, as ruxolitinib re-challenge may rapidly improve the clinical status of the patient. As mild or moderate RDS mainly occurred within a few days from discontinuation and affected > 10 % of patients, any other therapy should be started as close as possible to ruxolitinib discontinuation. RDS is often associated with an increase in spleen size and may therefore indicate residual JAK disease control activity, allowing to identify patients who could still benefit from second-line JAK2 inhibition.
A limitation of the study was that RDS prevention strategies were infrequent and inconsistent across the different centers. This may have prevented the detection of a correlation between tapering and RDS reduction. The implementation of consistent discontinuation strategies may allow for better RDS prevention in the future.
Expert opinion
For more information on how to manage RDS, watch our video with Francesca Palandri:
How do you manage ruxolitinib discontinuation syndrome?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Francesca Palandri
Francesca Palandri