All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
The long-term efficacy of interferon alpha in targeting different driver mutations involved in MPN
Mutations in JAK2V617F, calreticulin (CALR), and thrombopoietin receptor (MPL) genes are three main driver mutations associated with the pathogenesis of myeloproliferative neoplasms (MPN), where abnormality in hematopoietic stem cells (HSC) results in hyperplasia of myeloid lineages. Interferon alpha (IFNα) has been associated with complete hematological response rate (> 70%) and substantial molecular response (> 50%) in patients with MPN, however, low rate of complete molecular response remains a concern. Amandine Tisserand et al.1 have investigated the long-term efficacy of IFNα therapy by observing allele burden in HSC and mature cells of patients with JAK2V617F and CALR mutations to identify the pattern of response in an observational, prospective study. The results were presented by Tisserand during the Virtual Edition of the 25th European Hematology Association (EHA) Annual Congress.
Study design1
- Allele burden was assessed for 4 years, at intervals of 4 months, in patients with MPN
- in granulocytes and platelets from peripheral blood (mature cells)
- in different progenitors, including CD34+ CD38+ (common myeloid progenitors), CD34+ CD38− CD90− (immature progenitors), and CD34+ CD38− CD90+ (immature progenitors enriched in HSC)
- Molecular responses were defined as follows:
- Complete response: 100% decrease in mutant cells
- Partial response: > 50% decrease in mutant cells
- Minor response: 20–50% decrease in mutant cells
- No response: < 20% decrease in mutant cells
- The total number of patients was 50
- The most common types of MPN are polycythemia vera (PV; 50%), essential thrombocythemia (ET; 40%), and primary myelofibrosis (PMF; 10%)
- JAK2V617F was the most common driver mutation, present in 68% of patients, followed by CALR mutations (28%) and MPL mutations (4%)
Results1
The median follow-up for patients with a JAK2V617F mutation and patients with CALR mutations was 53 months and 42 months, respectively. The proportions of patients achieving molecular responses to IFNα in granulocytes and different progenitors are shown in Figure 1.
- In patients with a JAK2V617F mutation, complete molecular response rates were more frequent in the immature progenitors compartment (31–40%) compared with the mature granulocyte compartment (5%). The same pattern applied to partial response rates
- In patients with CALR mutations, complete and partial molecular response rates remained less than 15% in all hematopoietic compartments, with more than 70% of patients not showing a molecular response at all
Figure 1. Molecular responses to IFNα in granulocytes and different progenitors1
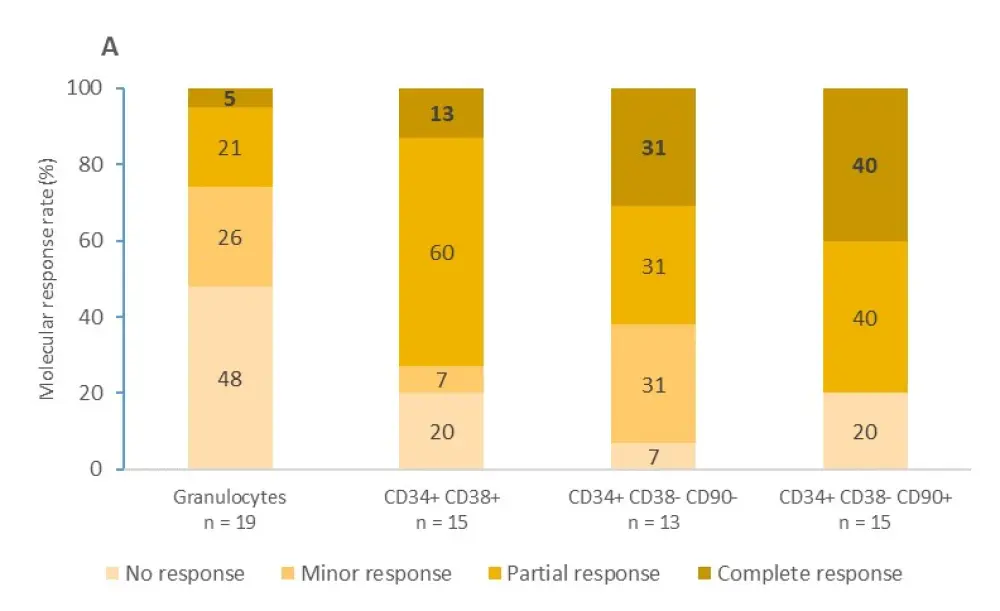
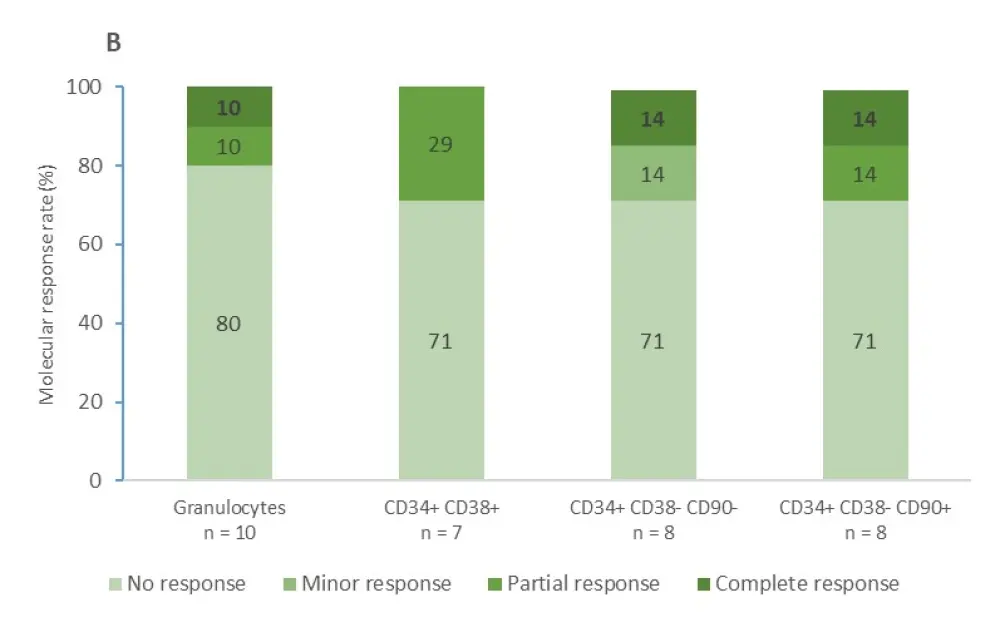
A Molecular response in patients with a JAK2V617F mutation B Molecular response in patients with a CALR mutation. Complete molecular response is expressed in bold.
The analysis of kinetics of JAK2V617F- and CALR-mutated cells showed the following:
- Over time, average allele burden decreases in patients with JAK2V617F treated with IFNα, and this decrease seems to be more rapid in progenitors compared to mature cells
- Interestingly, homozygous JAK2V617F progenitors demonstrated an important increase during the first year of IFNα treatment, followed by a substantial decrease thereafter
- In contrast, average allele burden in IFNα-treated patients with CALR mutations increased over time in both progenitors and granulocytes
Based on the mathematical model incorporated to define mechanism of action, IFNα is likely to show action by exhaustion of JAK2V617F homozygous HSC via differentiation into progenitors and then mature cells, which may explain the increase in the first year and the slow decrease thereafter.
It has been found that even low doses of IFNα were effective in targeting homozygous JAK2V617F HSCs, however, high doses (> 100 µg/week) were needed for heterozygous JAK2V617F HSCs. IFNα was least effective in targeting heterozygous CALR-mutated HSCs.
Conclusion
The study results suggest that IFNα can be effective in eliminating mutated HSCs and may be associated with better responses in patients with a homozygous JAK2V617F mutation, compared with heterozygous JAK2V617F or CALR mutations, when used in high doses. The increase in hematopoietic compartment during the first year may be predictive of efficacy. The authors believe that this study could be helpful to identify patients with MPN who may benefit from IFNα therapy.
Further investigation is planned to predict the IFNα response in patients based on allele burden prior to treatment, to explain the impact of other parameters, including related mutations, age, allele burden prior to treatment on the evaluation of long-term efficacy, and to discover the mechanism of action of IFNα therapy.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

