All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
The real-world survival benefit of ruxolitinib for myelofibrosis
The survival benefit of the Janus kinase (JAK) 1/2 inhibitor ruxolitinib for patients with myelofibrosis (MF) is well established in the COMFORT-I (NCT00952289), and COMFORT-II (NCT00934544) clinical trials. However, following approval from the U.S. Food and Drug Administration (FDA) in 2011 for the treatment of adult patients with intermediate- to high-risk primary and secondary or secondary MF, data have been limited on whether this survival benefit translates into the real-world setting. Previous real-world data, summarized on our hub, revealed a 47% reduction in the risk of mortality of patients with primary MF following ruxolitinib approval. However, survival comparisons based on exposure to ruxolitinib in the post-approval period are warranted in addition to further data comparing overall survival (OS) between the pre- and post-approval timeframes.
A retrospective study, recently published by Verstovsek et al.1 in Annals of Hematology, was performed using data from the US Medicare ‘Fee-for-Service’ claims database to examine the real-world impact of ruxolitinib in patients with MF. We summarize the key results below.
Study design
This was a retrospective study that collected data on patients with ≥1 inpatient or ≥2 outpatient claims with a diagnosis of intermediate- to high-risk MF from January 2010 through December 2017.
Eligible patients included those aged ≥65 years with intermediate-1 or higher risk MF, with a minimum of 12 months of pre-index continuous medical and pharmacy enrollment. Any patients diagnosed with other hematologic malignancies, or solid tumors either ≤12 months before, on, or any time after index were excluded.
Patients were divided into three categories:
- Pre-approval ruxolitinib-unexposed (n = 278), data from 2010–2011
- Post-approval ruxolitinib-unexposed (n = 1,127), data from 2012–2017
- Post-approval ruxolitinib-exposed (n = 272), data from 2012–2017
Results
Patient characteristics for the three cohorts are summarized in Table 1 below. Total number of patients included in the analysis was 1,677.
Table 1. Patient characteristics*
|
CCI, Charlson Comorbidity Index; ET, essential thrombocythemia; PV, polycythemia vera; SD, standard deviation. |
|||
|
Characteristic |
Pre-approval ruxolitinib-unexposed |
Post-approval ruxolitinib-unexposed |
Post-approval ruxolitinib-exposed |
|---|---|---|---|
|
Age, median (range) |
80.7 (65–102) |
78.0 (65–105) |
75.4 (65–94) |
|
Female sex, % |
70.1 |
58.7 |
55.9 |
|
Race, % |
|||
|
White |
84.9 |
82.9 |
88.2 |
|
Black |
8.6 |
10.2 |
4.8 |
|
Other/unknown |
6.5 |
6.9 |
7.0 |
|
History of PV, % |
12.2 |
6.8 |
20.2 |
|
History of ET, % |
15.1 |
15.9 |
19.5 |
|
CCI score, mean (SD) |
3.7 (2.7) |
3.2 (2.9) |
2.2 (2.2) |
|
Duration of follow-up, median, months, |
12.5 |
10.2 |
14.0 |
OS
- The 1- and 2-year survival rates for ruxolitinib exposure groups are summarized in Figure 1. The 1-year survival rate was greater in patients post-ruxolitinib approval compared with those in the pre-approval period irrespective of ruxolitinib exposure.
- However, when comparing survival rates in the ruxolitinib post-approval timeframe, both 1- and 2-year survival rates were greater in patients exposed to ruxolitinib.
Figure 1. 1- and 2-year survival rates for ruxolitinib exposure groups*
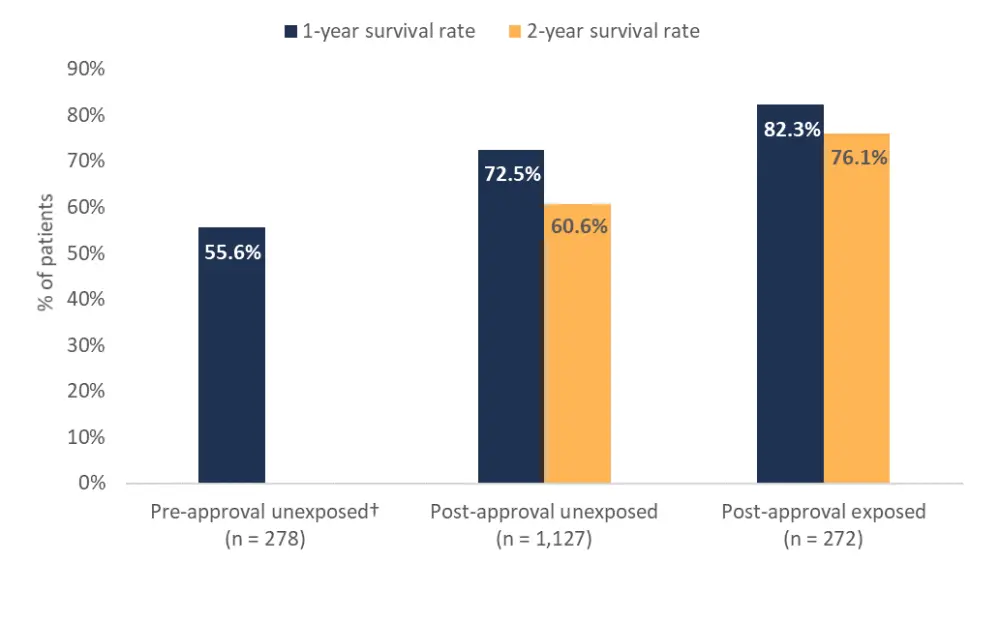
*Adapted from Verstovsek et al.1
†Data was not available for the 2-year survival rate in pre-approval unexposed patients.
- Median OS was 13.2 months (95% CI, 10.2–17.1) in the pre-approval unexposed group, 44.4 months (37.3–62.0) in the post-approval unexposed group, and not reached (95% CI, 51.0–NR) in the post-approval exposed cohort.
- Risk of mortality was significantly lower in both post-approval exposed (HR, 0.36, 95% CI, 0.26–0.50; p < 0.001) and post-approval unexposed (HR, 0.67; 95% CI, 0.56–0.80; p < 0.001) cohorts compared with the pre-approval group.
- Comparing the risk of mortality between post-approval exposure groups confirmed the survival benefit of ruxolitinib, with exposure to ruxolitinib associated with significantly reduced mortality risk (HR, 0.61; 95% CI, 0.45–0.83; p = 0.002).
Conclusion
Data from the real-world setting compliments clinical trial evidence for improved OS in patients with MF who receive ruxolitinib. Additionally, OS significantly improved from the pre-approval to post-approval time period regardless of ruxolitinib exposure, perhaps owing to increased disease awareness, and improved patient management over time. Between 2010 and 2017, treatment guidelines from the European LeukemiaNet and the World Health Organization (WHO) have been updated to include the use of ruxolitinib for the treatment of MF, and the use of several prognostic models for MF (e.g., International Prognostic Scoring System [IPSS]) have improved risk stratification.
Limitations of this study resulted from its retrospective design; as such, some clinical characteristics (e.g., hemoglobin level, circulating blast percentage, symptoms) were not available. Also, there was no comparison of patient characteristics at index, and differences may influence survival outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

