All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
The role of JAK2V617F allele burden in PV
Do you know... The increase of the JAK2V617F allele burden over time is a dynamic and time dependent factor for patients with PV. Which of the factors listed below is not associated with a variant allele frequency (VAF) increase overtime?
Polycythemia vera (PV) is a hematopoietic stem cell neoplasm, most often caused by a cytogenetic abnormality in the Janus kinase 2 (JAK2) gene.1 PV is characterized by an increased production of red blood cells, platelets, and neutrophils, resulting in disease-specific symptoms and an increased risk of vascular events and progression to myelofibrosis or acute myeloid leukemia.1 Current conventional treatments focus on the normalization of blood counts in order to control symptoms; however, several prospective clinical trials have demonstrated the potential of some therapies to achieve both blood count stabilization and a decrease in JAK2 mutant allele burden.1
Recently, Moliterno et al.1 published a review in Blood investigating the role of JAK2V617F allele burden in PV clinical outcomes as well as highlighting the prospective clinical trials where JAK2V617F burden is measured. We summarize the key points in the article below.
JAK2 and PV
- The JAK2 gene acts as a signal transducer for growth and differentiation in the hematopoietic stem cell compartment.
- The activating mutation commonly identified in patients with PV leads to an increase in signal transduction.
- Mutant JAK2 allelic burden can range from 0% to 100%. (Figure 1)
- A higher variant allele frequency (VAF) is associated with several physiological changes (Figure 1).
Figure 1. Quantitative, qualitative, and clonal burden of JAK2V617F*
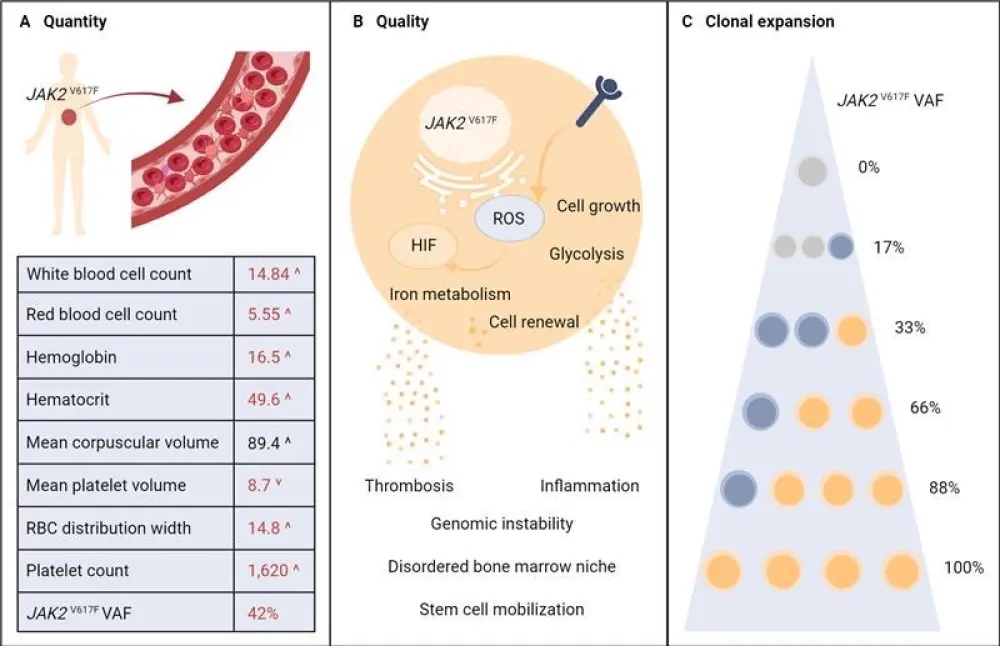
HIF, hypoxia inducible factor; ROS, reactive oxygen species; VAF, variant allele frequency.
*Adapted from Moliterno, et al.1
JAK2 and thrombotic risk
- The JAK2V617F and its VAF have an impact on both the quantity and quality of red blood cells produced by bone marrow.
- VAF >50% is independently associated with a significantly higher risk of venous thromboembolism, in both low- and high-risk patients.
- Other hematologic factors associated with a VAF include:
- Higher neutrophil to lymphocyte ratio
- Increased carotid plaque burden
- Higher elevations of C-reactive protein
Increase of variant allele frequency over time
- Clonal expansion due to increasing JAK2 VAF over time is a dynamic and time dependent factor.
- The recent PROUD-PV study (NCT01949805) estimated a VAF increase of 7% over 5 years.
- An increasing VAF over time has been found to be associated with:
- Prolonged disease duration
- Increased risk of disease progression
- Higher levels of lactate dehydrogenase
- Frequent occurrence of splenomegaly
- The average VAF in recently diagnosed low-risk patients was 35% in the LOW-PV trial (NCT03003325), compared to 75% for high-risk patients enrolled in the RESPONSE-1 trial (NCT01243944).
VAF and treatment directions
- The current goal of contemporary treatment is to prevent thrombotic events, improve PV-related symptoms, and prevent progression to myelofibrosis or acute myeloid leukemia.
- The ongoing approach involves regular phlebotomy or the administration of the cytoreductive agent hydroxyurea (HU) for the removal of excess circulating blood cells and lower hematocrit levels.
- Conversely, in recent real-world studies, both phlebotomy and HU have failed to demonstrate the benefit of normalizing blood cell counts in reducing thrombotic risk.
- Ruxolitinib showed greater hematocrit control and a higher probability of blood count stabilization vs the best available therapy in both the RESPONSE-1 and RESPONSE-2 (NCT02038036) trials.
- Ropeginterferon (ropegIFN) therapy showed higher complete hematologic responses at 24, 36, and 60 months compared with standard therapy of HU in the PROUD-PV and CONTINUATION-PV (NCT02218047) trials.
- Overall, superior blood count control is associated with reduced thrombotic risk; however, an extended period of time is required to observe a significant impact.
Prospective clinical trials
- Treatment with HU has been shown to reduce VAF in the first year but rebounds and rises after two years, with an increase of 1.2% per year.
- In contrast, both ruxolitinib and ropegIFN therapy have shown sustained reductions.
- The RESPONSE-1 trial recorded a decrease from 75% to 47% VAF over 5 years with ruxolitinib.
- Several trials investigating ropegIFN treatment have recorded VAF decreases from around 45% to as little as 3%.
- A reduction in VAF is associated with superior complete hematologic response and spleen response.
Conclusion
The knowledge of the JAK2V617F mutation in relation to PV has allowed both physicians and patients to understand that risk is not inherently based on blood counts alone but also due to the effects of JAK2V617F VAF on thrombosis and disease progression. Prospective trials are now highlighting the benefits of VAF reduction on clinical outcomes as well as certain treatment options that have a greater effect on reducing the allelic burden. However, there is still uncertainty around the degree of VAF to consistently reduce thrombotic risk, how closely peripheral VAF suppression reflects clonal suppression of JAK2-mutated cells, when to initiate therapy, and the durability of suppression after achieving reductions. These questions provide an exciting way forward to further explore superior disease-modifying therapies and better long- term clinical outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

