All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Timeline of key drug approvals in MPN treatment
Over the past two decades, the therapeutic landscape of myeloproliferative neoplasms (MPN) has rapidly evolved in conjunction with innovative research and pioneering clinical trials. Identification of the key driver mutation in the JAK2 gene represented a milestone in the development of disease-specific therapies. As a result, patients now have access to four Janus kinase inhibitors for the treatment of myelofibrosis, with momelotinib most recently approved in September 2023. Furthermore, interferon treatments and specific cytoreductive therapies have improved clinical outcomes for patients diagnosed with essential thrombocythemia and polycythemia vera.
To showcase the progression of treatment options over the past 20 years, we present a visual summary of approvals for common therapies used in MPN treatment (Figure 1).
Figure 1. Timeline of drug approvals in MPN
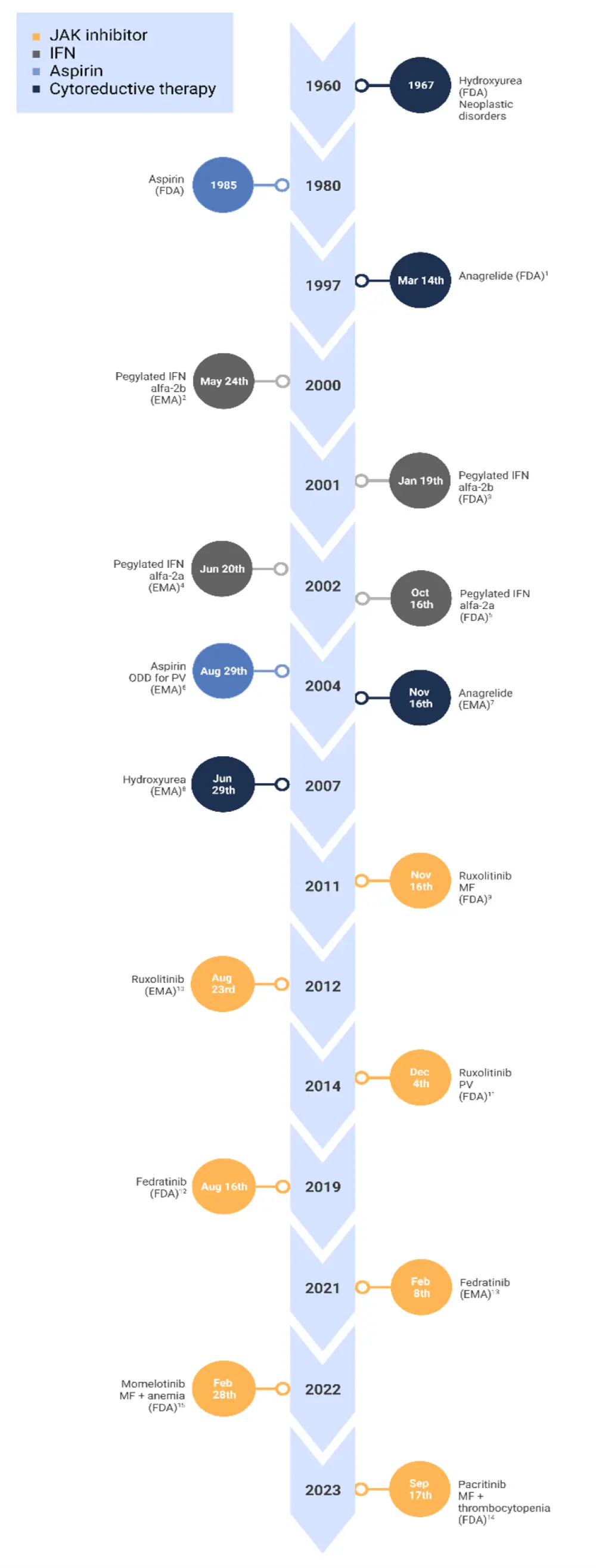
1 March 14th 1997, 2 May 24th 2000, 3 January 19th 2001, 4 June 20th 2002, 5 October 16th 2002, 6 August 29th 2004, 7 November 16th 2004, 8 June 29th 2007, 9 November 16th 2011, 10 August 23rd 2012, 11 December 4th 2014, 12 August 16th 2019, 13 February 2021, 14 February 28th 2022, 15 September 15th 2023
EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; IFN, interferon; JAK, Janus kinase; MF, myelofibrosis: OOD, orphan drug administration; PV, polycythemia vera.
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

