All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Allogeneic hematopoietic stem cell transplantation for myelofibrosis
Primary myelofibrosis (MF) is a type of myeloproliferative neoplasm (MPN) characterized by cytopenia, and splenomegaly, and can lead to leukemic transformation to acute myeloid leukemia (AML). The disease course can vary from indolent to severely symptomatic and highly progressive; thus, the median survival ranges from a few months to many years. Currently, various tools are available to stratify patients according to risk of mortality, including patient-related clinical characteristics, and molecular genetic profiling. The treatment for primary MF has evolved over the years. For instance, the introduction of Janus Kinase (JAK1/2) inhibitors, such as ruxolitinib, have revolutionized the outcome of patients; however, some patients still experience disease progression or relapse, and therefore, require transplantation.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) offers a curative treatment option for patients with primary MF and secondary MF (including post-essential thrombocythemia [ET]/polycythemia vera [PV]); nevertheless, there is a high risk of mortality and morbidity associated with allo-HSCT. Consequently, it is important to improve patient selection for transplantation.
A review by Malvi Savani and colleagues1 aimed to discuss the role of allo-HSCT in patients with MF, the optimal timing of allo-HSCT, novel distinctions in treatment considerations including the role of JAK2 inhibitors both pre- and post-allo-HSCT, the role of pre-HSCT splenectomy, new developments in optimal donor selection, and advances in conditioning regimens. This review was published in the British Journal of Haematology and is summarized below.1
Risk stratification
There are several risk classification systems that predict survival in primary MF (outlined in Table 1). Patients are classified into risk groups based on the number of factors involved in their disease: Low (0 factors); Intermediate-1 (1–2 factors); Intermediate-2 (3–4 factors); High (>4 factors). Patients with a low Dynamic International Prognostic Scoring System (DIPSS) score have a median life expectancy of 20 years, but for those with a high score, the median life expectancy is only 2–3 years. The presence or absence of certain mutations also affects the overall survival (OS) of patients with primary MF.
Table 1. MF risk classification systems*
|
DIPSS, Dynamic International Prognostic Scoring System; GIPSS, Genetically-Inspired Prognosis Scoring System; MF, myelofibrosis; MIPSS70-plus, Mutation Enhanced International Prognostic Score System for transplant age <70 years; RBC, red blood cells; WBC, white blood cells. |
|
|
Classification system |
Factors included |
|---|---|
|
DIPSS |
Age >65 years, |
|
DIPSS-plus |
All factors above plus: |
|
MIPSS70-plus |
All factors above plus: |
|
GIPSS |
Absence of type 1-like CALR gene mutation |
Impact of genetic mutations on allo-HSCT outcomes
Driver mutations in genes including JAK2, myeloproliferative leukemia virus oncogene (MPL), and CALR (variants Type 1 and Type 2) confer a selective proliferative advantage to cancer cells and result in variable clinical outcomes after allo-HSCT (as outlined in Table 2). Patients with ASXL1 mutations or triple-negative disease tend to have the worst outcomes.
Table 2. Mutations that affect outcomes of MF*
|
ET, essential thrombocythemia; GvHD, graft-versus-host-disease; MF, myelofibrosis; OS, overall survival; PFS, progression-free survival. |
||
|
Mutation |
Phenotype |
Outcome |
|---|---|---|
|
Type 1-like CALR |
MF with a high risk of progression from ET to secondary MF. |
2-year OS of 82% (vs 56% wildtype CALR). |
|
Type 2-like CALR |
ET with a low risk of thrombosis and indolent disease course. |
— |
|
JAK2 |
— |
Intermediate survival |
|
MPL |
— |
Intermediate survival |
|
JAK2−, MPL−, CALR− |
— |
Worst survival |
|
ASXL1 |
High risk of progression and leukemic transformation. |
Lower PFS than patients without the mutation |
Transplant considerations
- Patients with intermediate-1 or high-risk MF benefit from treatment with allo-HSCT in terms of OS; however, this is at the cost of early transplant-related mortality.
- The higher the DIPSS risk score, the greater the extent of long-term OS benefit with allo-HSCT.
- The risk of posttransplant complications is increased by extramedullary disease, portal hypertension, and pulmonary hypertension.
- Pulmonary hypertension because of extramedullary hematopoiesis is prevalent in ~50% of patients with MF; nevertheless, it is not routinely evaluated and diagnosed prior to allo-HSCT, despite there is evidence that it can negatively impact survival after allo-HSCT.
Role of pre-transplant splenectomy1
Splenomegaly has been linked to more frequent graft failures, increased risk of thrombosis, infections, and hemorrhage, which could delay or preclude patients with MF from undergoing HSCT.
Pre-transplant splenectomy in patients with MF has been linked with improved hematopoietic recovery and improved OS in some patients. However, as JAK2 inhibitors can generate a substantial reduction in splenomegaly, routine splenectomy prior to HSCT is questionable. Nonetheless, splenectomy can be considered in patients with massive splenomegaly who are refractory to ruxolitinib.
Ruxolitinib prior to transplant1
For most patients with MF, ruxolitinib has become the standard of care as it can provide a rapid, sustained reduction in splenomegaly, improve symptoms and quality of life, and may possibly increase survival. A recent study by the European Society for Blood and Marrow Transplantation-Chronic Malignancy Working Party (EBMT-CMWP), previously published on the MPN Hub, demonstrated that patients who responded to pretreatment ruxolitinib experienced a lower incidence of graft failure compared with those who experienced no response to ruxolitinib or those who had lost response. This suggests that transplantation while the patient is still responsive to ruxolitinib is preferable to waiting until a change in or loss of response.
However, the rate of drug discontinuation due to side effects or lack of efficacy is 38% after 1 year and 45% after 2 years. Moreover, clonal evolution occurs earlier and more frequently among patients on ruxolitinib compared with those who are not. Ruxolitinib may therefore exert a selective pressure on bone morrow cells, and so it is probably best to discontinue treatment if this occurs and consider either allo-HSCT or other investigational targeted agents.
Despite the above, tapering of ruxolitinib can lead to ‘ruxolitinib withdrawal syndrome’, which is characterized by rapid progression of disease, worsening of cytopenias, and rapidly increasing splenomegaly that can lead to hemodynamic instability, shock, and possible cytokine release syndrome. The tapering schedule is not standardized in the context of allo-HSCT, and schedules vary according to individual center protocols.
Some studies have shown that salvage treatment after ruxolitinib discontinuation can lead to clinical responses; nevertheless, these responses are rare and outcomes in this patient population are poor but can be improved by including allo-HSCT in the therapeutic strategy.
When should allo-HSCT be performed? 1
Biological age is no longer considered as a limiting factor for transplant; however, careful selection is necessary for patients aged over 65 years. Factors to consider when selecting patients for allo-HSCT include comorbidities (as they may limit the intensity of the transplant regimen and affect risk of relapse), Karnofsky index, clinical symptoms, quality of life, transfusion dependence, response to ruxolitinib, karyotype, and mutations.
A transplantation algorithm based on the DIPSS+ score can be seen in Figure 1.
- Patients with a low DIPSS+ score are generally not considered for allo-HSCT, while for those with a high score (>3), allo-HSCT is advised.
- Patients with an intermediate-1 risk (DIPSS+ score of 1–2) with ≥2% blasts in peripheral blood and/or transfusion dependence/refractoriness may also benefit from transplant.
- For patients with advanced disease, or those who do not respond to JAK inhibitors, allo-HSCT is the only viable option.
Figure 1. Transplantation algorithm for patients with MF based on the DIPSS+ score*
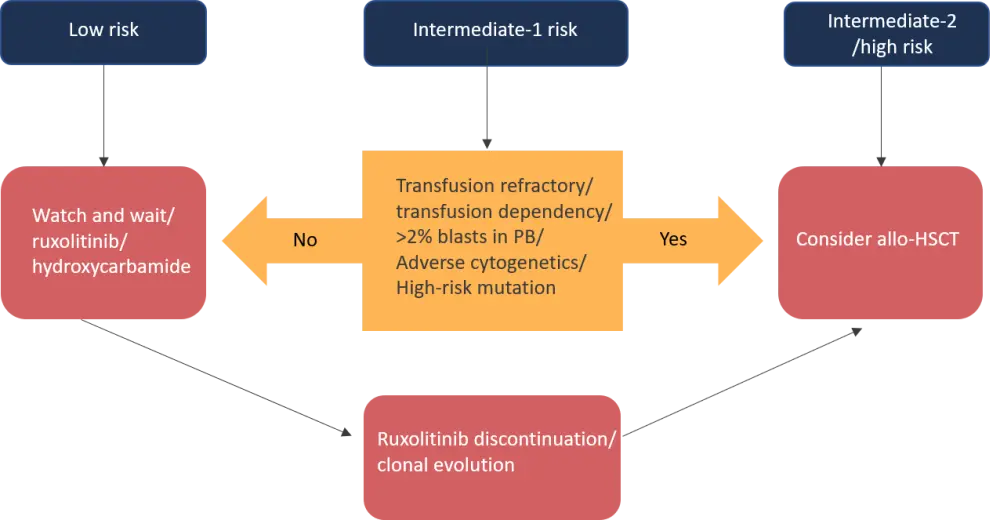
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; PB, peripheral blood.
*Adapted from Savani et al.1
Allo-HSCT is advised for patients with adverse karyotype or triple-negative disease (no mutations of JAK2, CALR or MPL1). Patients with an absence of Type 1 CALR mutations also have a similar risk profile to those with triple-negative disease, and so should be taken into consideration for early allo-HSCT. Allo-HSCT should also be considered for patients with ≥2 high-risk mutations and those with primary MF with any high-risk mutation, in addition to other clinical and transplant-specific risk factors.
Transplantation after progression/leukemic transformation1
Transformation to AML from MF or ET/PV occurs in ~10−20% of patients. For those with clonal evolution, with or without leukemic progression, transplantation is currently considered the best therapeutic option. Survival is not as good for patients who progress to leukemia compared with those transplanted earlier in the disease course. However, survival rates are better if patients are treated with chemotherapy and achieve complete remission (CR) prior to transplantation, compared with those that do not achieve CR.
Optimal donor selection1
In patients with MF, donor selection plays a significant role in the outcomes of allo-HSCT, with a lower non-relapse mortality (NRM) and better survival rates seen in patients with matched sibling donors compared with those with unrelated donors (URD) at 1 year. For those without a human leukocyte antigen (HLA)-identical related donor, URD cord blood and URD bone marrow are viable options; nevertheless, close monitoring is needed due to a high risk of NRM with URD cord blood transplantation. When no matched related donor or matched unrelated donors are available, it is recommended to consider haploidentical HSCT as patients can achieve engraftment with acceptable incidence of graft-versus-host disease (GvHD), with encouraging progression-free survival (PFS) and OS.
Conditioning regimen1
The type of conditioning regimen can impact posttransplant outcomes in terms of survival, relapse, and engraftment. Myeloablative conditioning regimens are not often used as patients with primary MF have a median age of 60−67 years of age, and so often have significant comorbidities. Therefore, they are given reduced toxicity (RTC) or intensity (RIC) conditioning regimens.
Current guidelines recommend the use of oral busulfan with targeted dosing according to plasma levels and alternatively, considering intravenous busulfan guided by plasma levels. Currently, there is no conclusive evidence to support the use of a particular conditioning regimen. Despite this, favorable results have been seen with the use of busulfan-cyclophosphamide and fludarabine-busulfan, and anti-thymocyte globulin (ATG).
For more information on conditioning prior to HSCT, watch our video with Craig W. Freyer, University of Pennsylvania, Philadelphia, US, at the 2021 Transplantation & Cellular Therapy Meetings of the American Society for Transplantation and Cellular Therapy (ASTCT) and the Center for International Blood and Marrow Transplant Research (CIBMTR), where he discusses various trials investigating conditioning regimens in this patient population.
Poor graft function and failure1
Poor graft function is defined as persistent severe neutropenia, with anemia and/or thrombocytopenia. Poor graft function remains a problem for a successful HSCT despite improvements in patient selection, timing of transplant, and conditioning strategies, as it occurs in 2−27% of patients with MF. For patients with full donor chimerism, an effective treatment for restoring normal graft function is a posttransplant CD34+-selected stem cell 'boost', which allows hematopoietic recovery in most patients without increasing the risk of GvHD, and avoids the use of aggressive toxic strategies, and a second transplant. For patients with mixed chimerism, new approaches need to be developed in prospective trials to enable sustained engraftment, lower NRM, reduce relapse, and improve survival and quality of life.
Graft failure occurs in 2−24% of patients with MF who undergo HSCT. Factors that contribute to graft failure include: type of conditioning regimen used; alternative donor selection (mismatched unrelated donor, cord blood donor or haploidentical sources); inadequate number of CD34+ cells infused; negative donor/recipient cytomegalovirus serostatus; the degree of splenomegaly, and the degree of fibrosis and thrombocytopenia prior to transplant.
Role of posttransplant ruxolitinib1
Data regarding the efficacy of ruxolitinib as post-HSCT maintenance for patients with MF are sparse. Nevertheless, a pilot retrospective study conducted by Pu et al.,2 which evaluated the feasibility and toxicity of ruxolitinib regimens both pre-HSCT and post-HSCT as maintenance, demonstrated that 5 mg ruxolitinib twice daily post-HSCT can serve as a potential novel approach for GvHD prophylaxis, and potentially achieve a greater degree of spleen size reduction, improved resolution of fibrosis, and rapid engraftment. Despite this, further large-scale prospective studies are needed before it can be implemented as standard of care for this patient population.
Role of posttransplant cyclophosphamide1
Although the use of posttransplant cyclophosphamide (PTCy) has revolutionized haploidentical and unrelated allogeneic HSCT outcomes in terms of decreasing the incidence of acute and chronic GvHD, there is sparse data of its use in the MF setting. A recent prospective pilot trial conducted in Italy (previously summarized on the MPN Hub) evaluated GvHD prophylaxis with PTCy and ruxolitinib in 20 patients with primary or secondary MF. The trial demonstrated that prophylaxis with these agents resulted in a low toxicity profile, with 30% of patients experiencing Grade 3−4 nonhematologic toxicity, 45% viral reactivation, and 15% severe sepsis. There was also low relapse incidence and good acute and chronic GvHD control. Two-year NRM, OS, and event-free survival were 15%, 85%, and 72%, respectively. The use of both ATG and PTCy, investigated in another trial,3 also showed a high OS (74.4% at 1 year), relapse-free survival (71.3% at 1 year), and a low incidence of acute and chronic GvHD.
Relapse post-allogeneic HSCT1
Treatments for patients who experience relapse after HSCT include early withdrawal and reduction of posttransplant immunosuppression, administration of donor lymphocyte infusion, and a second HSCT. Donor lymphocyte infusions have been successfully used for mixed chimerism or relapse, either pre-emptively or as salvage therapy, and have been established as a safe and effective strategy for patients with residual disease. Data on a second HSCT in relapse are sparse, although a recent study of treosulfan in combination with fludarabine and ATG conditioning before second HSCT,4 showed successful leukocyte engraftment by a median of Day 11 in all patients, acute Grade II–IV GvHD at Day 100 in ~56% of patients, and an acceptable toxicity profile with a 5-year disease-free survival and OS of 45% and 47%, respectively.
Conclusions
The risks of a patient's disease course as well as outcomes following HSCT can be prognosticated by various clinical, patient-related, and molecular components. Certain mutations such as ASXL1 and triple-negative disease status, confer a higher risk of relapse and mortality, and therefore, patients exhibiting these features should be considered for early transplant as should patients with clonal evolution and lack of response to JAK inhibitors. Other important factors for consideration include comorbidities, clinical symptoms, and quality of life.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

