All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Novel agents to treat MF with suboptimal response to ruxolitinib: Long-term outcome with momelotinib
Featured:
Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway dysregulation is central to the pathogenesis of myelofibrosis (MF), giving rise to progressive anemia, splenomegaly, and other, constitutional symptoms in patients with MF.1 The JAK1/JAK2 inhibitor (JAKi), ruxolitinib, was approved for the treatment of patients with primary and secondary intermediate-/high-risk MF in 2011, based on results showing significantly reduced spleen volume, improved MF-related symptoms and quality of life, and prolonged overall survival (OS).2 However, there remains an unmet clinical need for the effective management of patients with MF who need to discontinue JAKi therapy due to toxicity, as OS after JAKi discontinuation is short.
During the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, there were a number of presentations on the novel agents and treatment combinations under evaluation for patients with MF. The MPN Hub is happy to present a summary on the JAK1/JAK2 and activin A receptor type I (ACVR1) inhibitor, momelotinib, which has been proposed as a potentially promising treatment option for patients with MF, particularly those experiencing hematological toxicity and myelosuppression during JAKi therapy. At ASH 2020, Srdan Verstovsek, University of Texas MD Anderson Cancer Center, discussed the long-term outcome data from the phase III SIMPLIFY-1 (NCT01969838) and SIMPLIFY-2 (NCT02101268) trials evaluating momelotinib for patients with JAKi-naïve and JAKi-treated MF, respectively.1
Study design
- SIMPLIFY-1 and SIMPLIFY-2 (Figure 1) were non-inferiority and superiority studies, respectively, and each shared the same study endpoints:
- Primary – splenic response rate at Week 24
- Secondary – total symptom score and transfusion independence (TI) rate
- Patients were initially randomized to receive ruxolitinib (best available treatment [BAT] was used in SIMPLIFY-2) or momelotinib. Open label momelotinib was available to all patients from Week 24 as shown in Figure 1.
- Previous data have suggested that momelotinib improves constitutional symptoms, reduces transfusion burden and anemia, and has a favorable safety profile.3,4
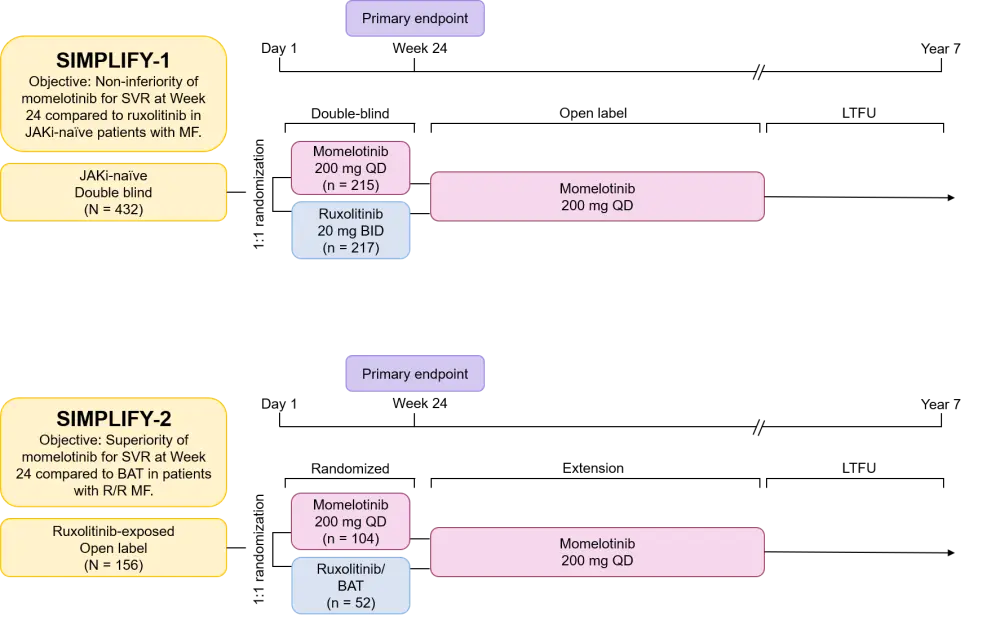
BAT, best available treatment; BID, twice daily; JAKi, Janus kinase inhibitor; LTFU, long-term follow-up; MF, myelofibrosis; QD, once daily; R/R, relapsed and/or refractory; SVR, spleen volume reduction.
Results
- Encouraging OS results were observed across both the SIMPLIFY-1 and SIMPLIFY-2 studies, irrespective of whether patients were initially randomized to ruxolitinib/BAT or momelotinib as shown in Table 1.
Table 1. OS of patients enrolled in SIMPLIFY-1 and SIMPLIFY-21
|
HR, hazard ratio; NR, not reached; OS, overall survival.*Patients crossed over from treatment with ruxolitinib (or BAT in SIMPLIFY-2) to momelotinib at Week 24. |
|||||
|
Trial |
Patient population |
Median OS, months |
HR |
p |
|
|---|---|---|---|---|---|
|
Ruxolitinib followed by momelotinib* |
Momelotinib† |
||||
|
SIMPLIFY-1 |
JAKi-naïve |
53.1 |
NR |
0.99 |
0.97 |
|
SIMPLIFY-2 |
Ruxolitinib-exposed |
37.5 |
34.3 |
0.96 |
0.86 |
- Further analysis of SIMPLIFY-1 showed:
- Stable hemoglobin and platelet levels during therapy allowed for full dose intensity treatment with momelotinib, whereas high rates of dose reductions were seen with ruxolitinib.
- Spleen response rates were 27% for momelotinib and 29% for ruxolitinib, demonstrating non-inferiority to ruxolitinib at Week 24 (p < 0.001). Furthermore, 40% of patients achieved a splenic response with momelotinib at any time. Splenic responses were durable irrespective of whether patients were initially randomized to ruxolitinib/BAT or momelotinib, and the median duration of response was not reached at > 3 years of follow-up.
- Landmark analysis of TI at 24 weeks showed better rates for momelotinib (67%) versus ruxolitinib (49%; p < 0.001). Median duration of TI response was not reached after 3 years.
Conclusion
Momelotinib demonstrated robust OS outcomes in both JAKi-naïve and JAKi-treated patients with MF that did not discriminate according to initial therapy. JAKi-naïve patients treated with momelotinib achieved splenic responses and sustained transfusion independence while showing only mild myelosuppression and no cumulative toxicity. This compares well with ruxolitinib, where thrombocytopenia-induced treatment interruption or discontinuation is more frequent.
The global effort to identify optimal therapies to manage patients with MF continues, and novel druggable targets and agents are being investigated in the MF setting. At ASH 2020, novel combination regimens with ruxolitinib were presented using navitoclax and KRT-232: read a summary here. For an overview of the novel agents for MF, read our review article here.
Additional resources
For more information on the long-term safety analyses of momelotinib in patients with MF, as determined in the SIMPLIFY-1/2 studies, click here.
During ASH 2020, the MPN Hub spoke to Steering Committee member Ruben Mesa, UT Health San Antonio, San Antonio, US, who discussed the long-term benefit of momelotinib in the SIMPLIFY-1+2 trials.
SIMPLIFY-1+2 trial: What is the long-term benefit of momelotinib treatment in R/R MF?
Watch the video below for an interview with Abdulraheem Yacoub, University of Kansas Medical Center, Kansas City, US, on the clinical value and potential of momelotinib for anemic patients with MF.
What is the clinical value and potential of momelotinib for anemic patients with myelofibrosis?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Ruben A. Mesa
Ruben A. Mesa Abdulraheem Yacoub
Abdulraheem Yacoub